Conjugated polymer based on indolinone and naphthalimide units and preparation method thereof
A naphthalene diimide and conjugated polymer technology, which is applied in the field of organic semiconductor acceptor polymers and their preparation, can solve the problems of few application reports of synthetic polymers, and achieve good chemical stability, low price, and easy availability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, preparation of conjugated polymer based on indolinone and naphthalimide unit conjugated polymer conjugated polymer
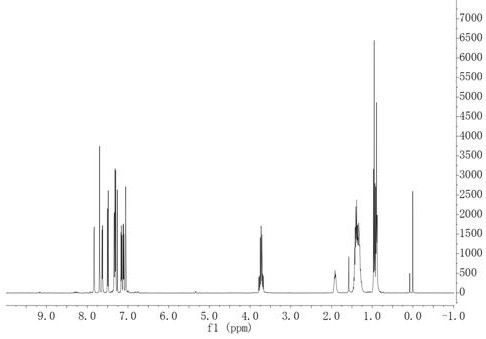
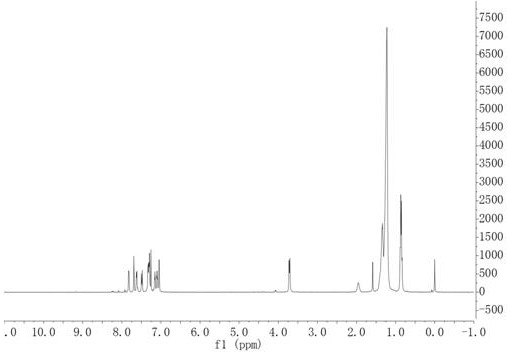
[0046] This example provides two kinds of soluble conjugated polymers based on indolinone and naphthalene diimide units, the structural formula of which is shown in Table 1 (wherein, n≥1), and its synthetic route can be found in figure 1 .
[0047]
[0048] 1.1. Preparation of monomer M1
[0049] The synthetic route of the first monomer M1 (M1-1, M1-2) of P1, P2 polymer is roughly the same, and it specifically comprises the following steps:
[0050] (a) Synthesis of intermediate compound C
[0051] The structural formula of intermediate compound C is
[0052]
[0053] Its detailed preparation method is: under the protection of nitrogen, put 6-bromo-2-indolinone (20mmol), 2-thiophene carboxaldehyde (22mmol) into a two-necked flask, and then add a certain amount of methanol, the reactant A suspension formed. Then measure 5ml of pipe...
Embodiment 2
[0075] Embodiment 2, the ultraviolet absorption spectrum and the electrochemical property of polymer P1
[0076] 2.1. UV absorption spectrum of polymer P1
[0077] Image 6 The ultraviolet absorption spectrum of polymer P1 in chloroform and film is given, and it can be seen that the polymer has two absorption peaks. In the film, NDI with side chains has a strong absorption at 350nm-550nm, and thiophene-2-indole Keto-thiophene has a strong absorption at 600nm-780nm, and generally speaking, it has a wide range of ultraviolet-visible light absorption. The onset absorption of the film (λonset film ) is 842nm, according to the formula Eg film =1240 / λonset film Eg can be obtained by eV calculation film =1.47eV, indicating that the polymer is a narrow bandgap polymer.
[0078] 2.2. Electrochemical properties of polymer P1
[0079] The electrochemical properties of the polymer were tested with platinum sheet as working electrode, platinum wire as counter electrode and saturated...
Embodiment 3
[0081] The ultraviolet absorption spectrum and electrochemical properties of embodiment 3, polymer P2
[0082] 3.1. UV absorption spectrum of polymer P2
[0083] Figure 7 The ultraviolet absorption spectrum of polymer P2 in chloroform and film is given, and it can be seen that the two absorption peaks of the polymer have similar absorption intensities. Therefore, polymer P2 has a wide range of UV-visible light absorption and high absorption intensity. The onset absorption of the film (λonset film ) is 845nm, according to the formula Eg film =1240 / λonset film Eg can be obtained by eV calculation film = 1.47eV.
[0084] 3.2. Electrochemical properties of polymer P2
[0085] The electrochemical properties of the polymer were tested with platinum sheet as working electrode, platinum wire as counter electrode and saturated calomel electrode as reference electrode. In anhydrous acetonitrile solvent, with the concentration of 0.1mol / L tetrabutylammonium hexafluorophosphate a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com