Quinoxaline-based D-A-pi-A type organic photosensitizer as well as synthesis method and application thereof
A photosensitizer, quinoxaline technology, applied in near-infrared organic photosensitizers and photosensitizer-based tumor photodynamic therapy applications, to achieve improved performance, easy access to raw materials, and good photodynamic therapy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
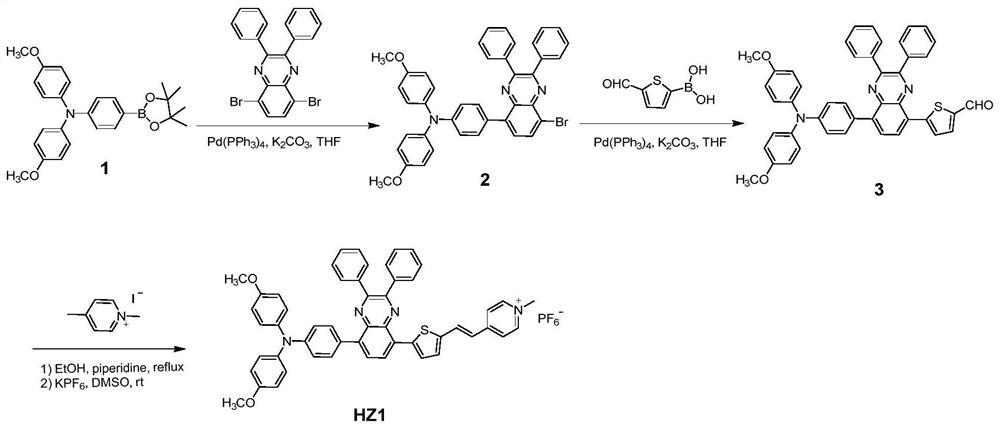
[0040] Synthesis of D-A-π-A Type Organic Photosensitizer HZ1 Based on Quinoxaline
[0041] (1) Synthesis of compound 2
[0042]
[0043] Under the protection of argon, in a 50mL two-necked round bottom flask, 862mg (2.0mmol) compound 1, 968mg (2.2mmol) dibromodiphenyl substituted quinoxaline, 3mL 2M K 2 CO 3 Aqueous solution was added to 20mL THF solvent, and then 115mg Pd(PPh 3 ) 4 catalyst. The reaction mixture was heated to 70 °C for 12 h. After cooling to room temperature, 30 mL of deionized water was added to the reaction liquid, and extracted several times with dichloromethane. The organic phase obtained by extraction and merging was dried with anhydrous magnesium sulfate and then rotary evaporated to remove the organic solvent, and the crude product was separated and purified by silica gel column chromatography, using normal hexane: dichloromethane (2:1) as mobile phase to obtain (0.97g ) orange solid compound 2, the yield was 73%. NMR: 1 H NMR (400MHz, CDCl ...
Embodiment 2
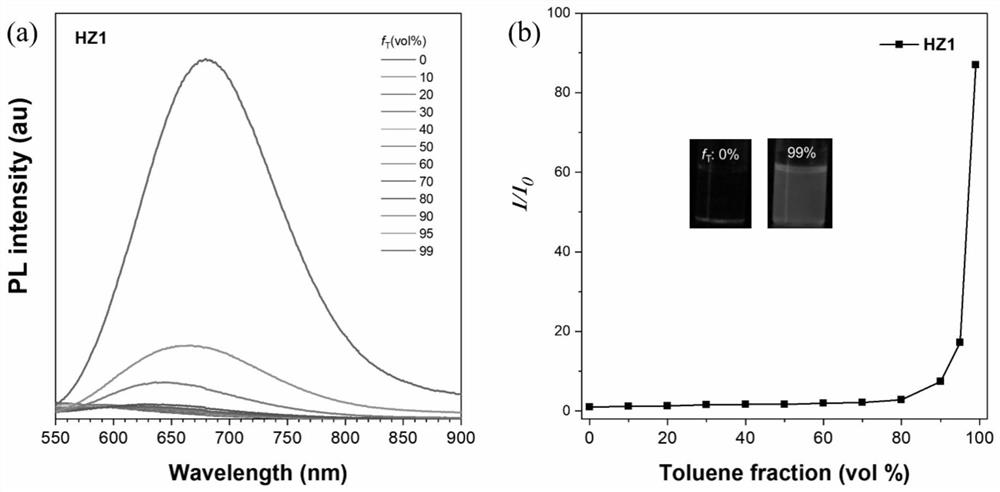
[0051] Photosensitizer HZ1 of embodiment 1 has been carried out AIE performance test, as figure 2 shown. The D-A-π-A type organic photosensitizer HZ1 based on quinoxaline was tested for its AIE performance in DMSO / toluene. It can be seen from Figure 2a and 2b that with the increase of the volume of toluene, the fluorescence intensity of the solution gradually increases, and when the volume of toluene accounts for 99%, the fluorescence intensity increases by 87 times. This shows that the photosensitizer has good AIE performance.
Embodiment 3
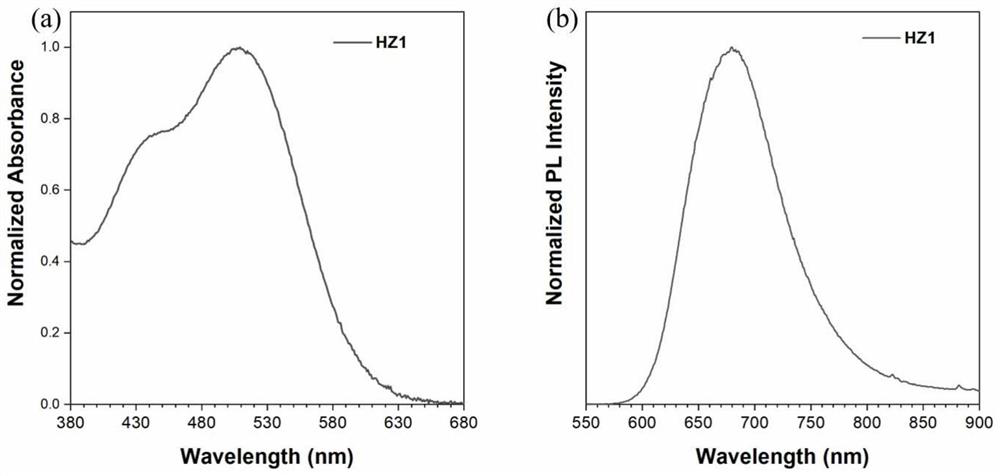
[0053] The photosensitizer HZ1 of Example 1 was tested by liquid ultraviolet and solid fluorescence. Such as image 3 shown. The UV-Vis absorption spectrum of dye HZ1 in DMSO is shown as image 3 As shown in a, the maximum absorption wavelength of the dye is 509nm, and the absorption spectrum covers most of the visible region. image 3 B is the solid-state fluorescence emission spectrogram of the dye, the fluorescence emission peak of the dye is at 679nm, and most of the areas on the right side of the dye are in the near-infrared wavelength region, which shows that the photosensitizer has very high near-infrared fluorescence emission performance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com