Oxazepam hapten, oxazepam antigen and preparation method and application thereof
A hapten and antigen technology, applied in the biological field, can solve the problems of being expensive, unable to meet the requirements of fast, convenient, and accurate, and achieve the effects of improving the detection rate, reducing cross-substance interference, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] This embodiment prepares a kind of oxazepam hapten, and the preparation method is as follows:
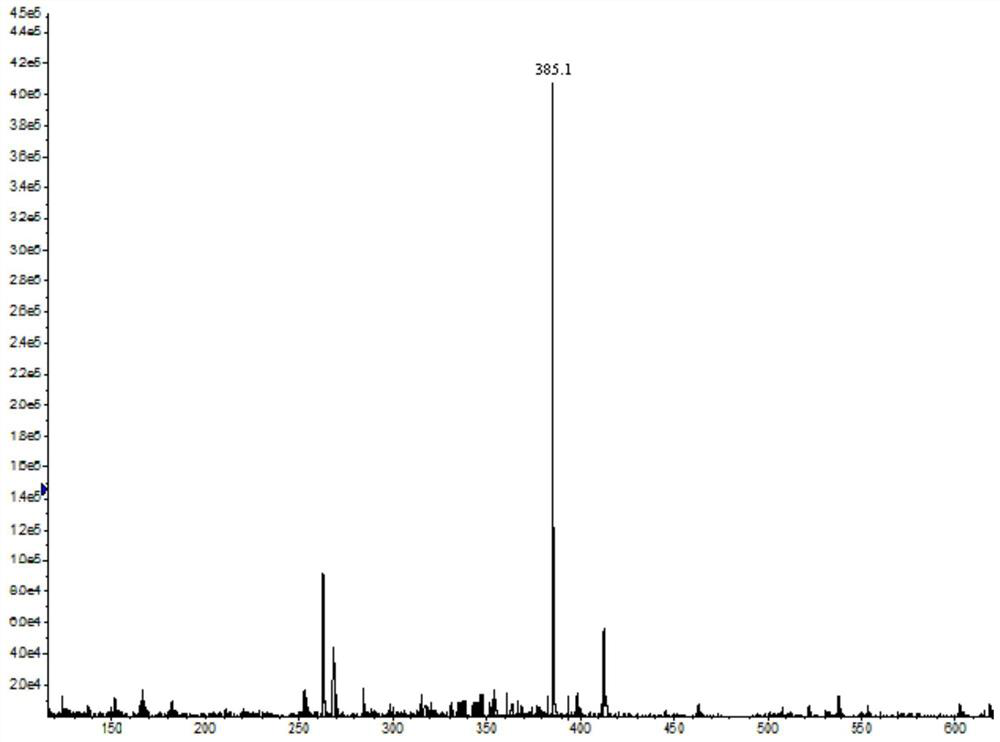
[0055] Weigh 10mmol of anhydrous aluminum trichloride and 5mmol of succinic anhydride in a 50mL round-bottomed flask, add 10mL of dichloromethane, and stir at 0°C; weigh 5mmol of oxazepam, and add in three times to the above reaction After the addition, it was raised to 25°C and reacted for more than 12 hours; under vigorous stirring, slowly added 20mL of ultrapure water, and after standing for stratification, the organic layer was taken for vacuum distillation, and the obtained residue was dissolved in 5ml of ethyl acetate. Wash twice with saturated sodium chloride solution, 5 mL each time, dry the organic layer, evaporate to dryness, and obtain 1.8 mmol hapten after purification by column chromatography. Carry out ESI-MS analysis (385.1[M-1]) to the prepared hapten, the result is as follows figure 1 As shown, it was proved that the oxazepam hapten was successfully prepared...
Embodiment 2
[0058] This embodiment prepares a kind of oxazepam hapten, and the preparation method is as follows:
[0059] Weigh 20mmol of anhydrous aluminum trichloride and 5mmol of succinic anhydride into a 50mL round-bottomed flask, add 10mL of dichloromethane, and stir at 0°C; weigh 5mmol of oxazepam, and add it to the above reaction in 3 times After the addition, it was raised to 25°C and reacted for more than 12 hours; under vigorous stirring, slowly added 20mL of ultrapure water, and after standing for stratification, there were a large amount of flocculent precipitates, and the organic layer was taken for vacuum distillation, and the obtained residual Dissolve in 5ml ethyl acetate, wash twice with saturated sodium chloride solution, 5mL each time, dry the organic layer, evaporate to dryness, and obtain 0.03mmol hapten after purification by column chromatography. The obtained hapten was compared with the hapten obtained in Example 1, and it was found to be the same substance, but th...
Embodiment 3
[0062] This embodiment prepares a kind of oxazepam antigen, and the preparation method is as follows:
[0063] (1) Preparation of oxazepam hapten active ester:
[0064] In a 25 mL dry single-necked bottle, add 1 mmol of the hapten prepared in Example 1, 1.2 mmol of N-hydroxysuccinimide, and 1.2 mmol of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride and 10mL N,N-dimethylformamide were stirred at 25°C for 18h under the protection of helium. After the reaction was completed, the reaction mixture was divided into eight 1.5mL centrifuge tubes, centrifuged at 10000rpm for 30min, and the supernatant was divided into eight 2mL glass sealed bottles and stored under the protection of helium gas for later use.
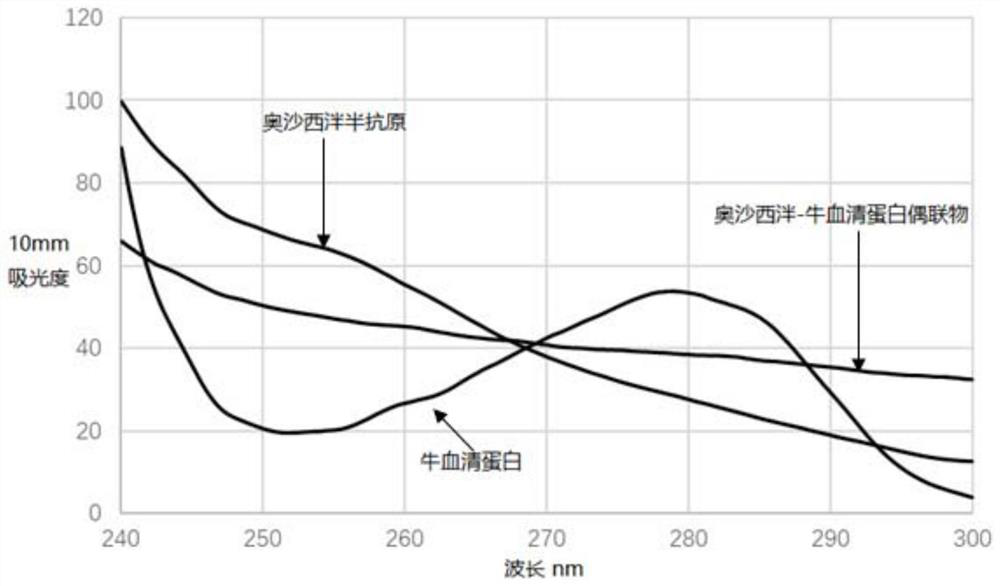
[0065] (2) Preparation of Oxazepam Antigen (Oxazepam-Bovine Serum Albumin):
[0066] Take 100mg BSA in a 25mL single-necked bottle, add 8mL of 0.1M sodium carbonate-sodium bicarbonate buffer solution with pH 9.6 and 1.4mL N,N-dimethylformamide and stir to dissolve,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com