Method for preparing pellets of chondrocytes from human induced pluripotent stem cells, and use thereof
A technology of pluripotent stem cells and chondrocytes, applied in the field of preparation of chondrocyte particles, can solve the problems of low differentiation speed, increased time, differentiation or deformation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
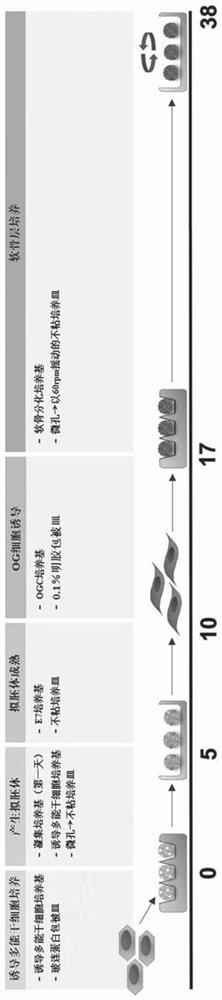
[0126] Preparation of Human Induced Pluripotent Stem Cells
[0127] Human induced pluripotent stem cells (hiPSC) were prepared from cord blood mononuclear cells (CBMC). CBMCs obtained from the cord blood bank of St. Mary's Hospital in Seoul, Korea were used. Cord blood was diluted with phosphate buffered saline (PBS), and centrifuged through a Ficoll gradient at 850×g for 30 minutes to collect CBMCs, washed, frozen and stored until use. After the CBMC were thawed before use, they were resuspended in StemSpan medium (STEMCELL Technological, Vancouver, British Columbia, Canada) to which CC110 cytokine cocktail (Cytokine cocktail; STEMCELL) had been added, and incubated at 37°C in 5% CO 2 Cultured in the incubator for 5 days.
[0128] The cultured CBMC were divided into 3×10 5 was inoculated into 24-well plates, and CBMC-derived hiPSCs were obtained by inducing reprogramming using the CytoTune™-iPS20 Sendai reprogramming kit (sendai reprogram kit; A16518, Life Technologies) ac...
Embodiment 2
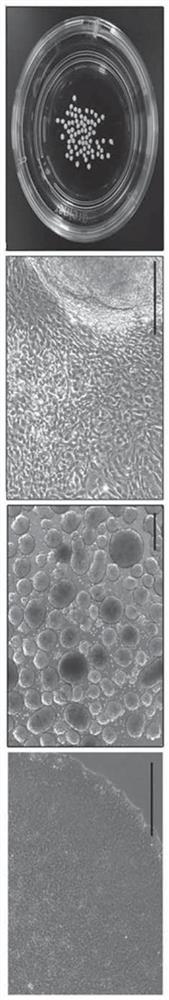
[0131] Formation of embryoid bodies from hiPSCs
[0132] The CBMC-derived hiPSCs prepared in the above Example 1 were resuspended in Aggrewell medium (STEMCELL) at 2×10 6 Concentrations of cells / well were seeded into 100-mm culture plates. The inoculated hiPSCs were cultured in a 37 °C incubator for 24 hours, and the next day the medium was replaced with TeSR-E8 medium (containing 543 μl / ml sodium bicarbonate (NaHCO 3 ), 64μg / ml of L-ascorbic acid 2-phosphate magnesium (L-Ascorbic acid2-phosphate magnesium), 14ng / ml of sodium selenite (Sodium selenite), 10 7 μg / ml of transferrin (Transferrin), 20 μg / ml of insulin, 100ng / ml of fibroblast growth factor-2 (FGF-2) and 2ng / ml of transforming growth factor beta 1 (transforming growth factor beta 1, TGF -β1) After adding glutamine (glutamine) and HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid)) to the DMEM / F12 medium, an additional 6 days of adherent culture was carried out to form and obtain pseudo embryoid body.
Embodiment 3
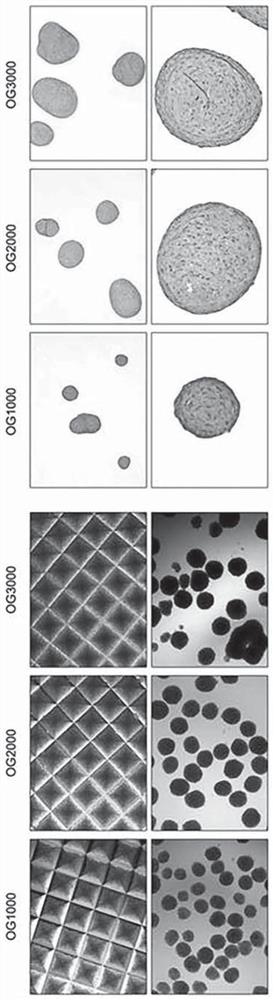
[0134] Formation and isolation of growing cells from embryoid bodies
[0135] The embryoid body (embryoid body, EB) formed and obtained in the above-mentioned Example 2 was suspended in DMEM medium (Thermo Fisher Scientific) containing 20% fetal bovine serum (Fetal Bovine Serum) and 10% penicillin / streptomycin, on gelatin-coated plates at 37°C in 5% CO 2 The formation of outgrowth cells (OG) was induced by culturing for 7 days. For this, culture plates were used which were completely dry after 30 minutes of coating the bottom with 0.1% gelatin.
[0136] Formed OG cells were isolated from gelatin-coated plates and passed through a 40 μm-sized cell strainer (cellstrainer; Thermo Fisher Scientific) to remove EB clumps, thereby isolating and obtaining only single-cell units of OG cells.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com