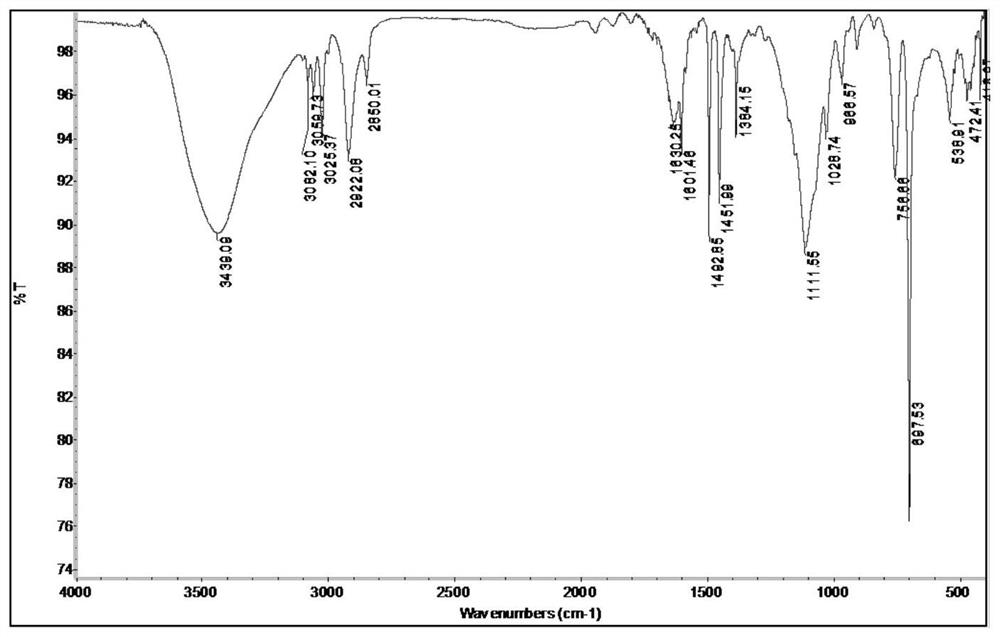
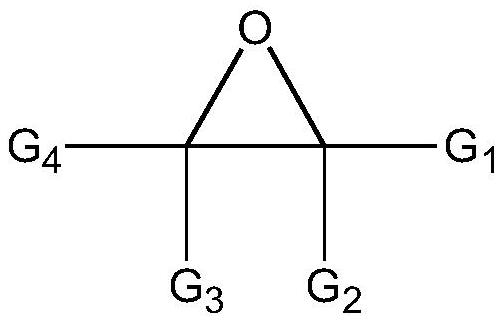
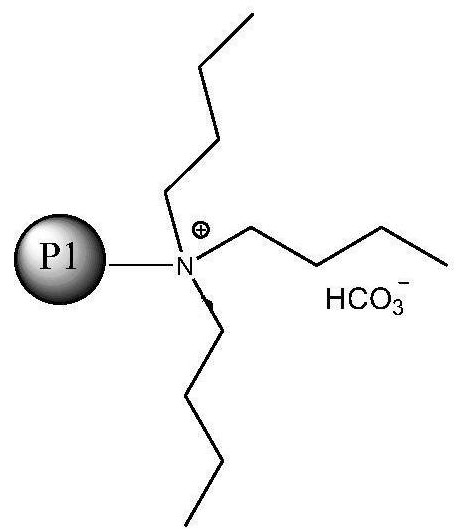
Nano composite ion exchange resin catalyst and application thereof
An ion exchange resin and nanocomposite technology, applied in the field of nanocomposite ion exchange resin catalyst, can solve the problems of low yield, harsh operating conditions, long synthesis route, etc., and achieve high catalyst activity, high conversion rate and high alkali resistance. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] [Example 1] Preparation of Ion Exchange Resin Catalyst
[0050] Add 65.0 g of styrene, 1.0 g of divinylbenzene, 3.0 g of octavinylsilsesquioxane and 1.0 g of benzoyl peroxide into a 500 ml three-necked flask, start the stirrer and stir for 0.5 hours; add 200 ml of deionized A mixed solution of water and 4 g of polyvinyl alcohol was stirred for 2 hours. Then gradually raise the temperature to 75°C, react for 5 hours, then raise the temperature to 90°C, react for 10 hours, and finally raise the temperature to 100°C, react for 10 hours. After the reaction, pour out the upper liquid, wash with 85°C hot water, then wash with cold water, then filter, put in an oven to dry at 80°C, sieve, and collect composite microspheres with a particle size within the range of 0.35 to 0.60 mm A1.
[0051] Chloromethylation: In a 500ml three-necked flask, add 50g of composite microspheres A1 and 200ml of chloromethyl ether, let it stand at room temperature for 6 hours, add 30g of zinc chlo...
Embodiment 2
[0055] [Example 2] Preparation of Ion Exchange Resin Catalyst
[0056] Add 50.0 grams of styrene, 1.6 grams of divinylbenzene, 4.5 grams of octavinylsilsesquioxane and 1.0 grams of benzoyl peroxide into a 500 milliliter three-necked flask, start the stirrer and stir for 0.5 hours; add 200 milliliters of deionized A mixed solution of water and 4 g of polyvinyl alcohol was stirred for 2 hours. Then gradually raise the temperature to 60°C, react for 5 hours, then raise the temperature to 90°C, react for 12 hours, and finally raise the temperature to 100°C, react for 12 hours. After the reaction, pour out the upper liquid, wash with 85°C hot water, then wash with cold water, then filter, put in an oven to dry at 80°C, sieve, and collect composite microspheres with a particle size within the range of 0.35 to 0.60 mm A2.
[0057] Chloromethylation: In a 500 ml three-necked flask, add 50 g of composite microspheres A2 and 200 ml of chloromethyl ethyl ether, let it stand at room tem...
Embodiment 3
[0061] [Example 3] Preparation of Ion Exchange Resin Catalyst
[0062] Add 50.0 grams of styrene, 2.6 grams of divinylbenzene and 1.6 grams of benzoyl peroxide initiator in a 500 milliliter three-necked flask, then add 0.6 grams of octavinylsilsesquioxane, and add 2.0 grams of gelatin that has been dissolved 260 ml of deionized aqueous solution was gradually heated up, and stirred and reacted at 60° C. for 2.0 hours. At the same time, the temperature was gradually raised to 80°C and reacted for 5 hours; then the temperature was raised to 90°C and reacted for 5 hours, and finally the temperature was raised to 98°C and reacted for 6 hours. After the reaction, pour out the upper liquid, wash it with 85°C hot water, then wash it with cold water, then filter, put it in an oven to dry at 80°C, sieve, and collect composite microspheres with a particle size within the range of 0.35-0.60mm A3.
[0063] Chloromethylation: In a 500ml three-necked flask, add 40g of composite microsphere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com