Synthesis method of thionyl diacetic acid
A technology of thiomethylene diacetic acid and a synthetic method, which is applied in the direction of mercaptan preparation, sulfide preparation, electrolysis process, etc., can solve the problems of hydrogen sulfide poisonous gas, environmental pollution, yield decline, etc. Effect of reducing pollution and reducing raw material consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
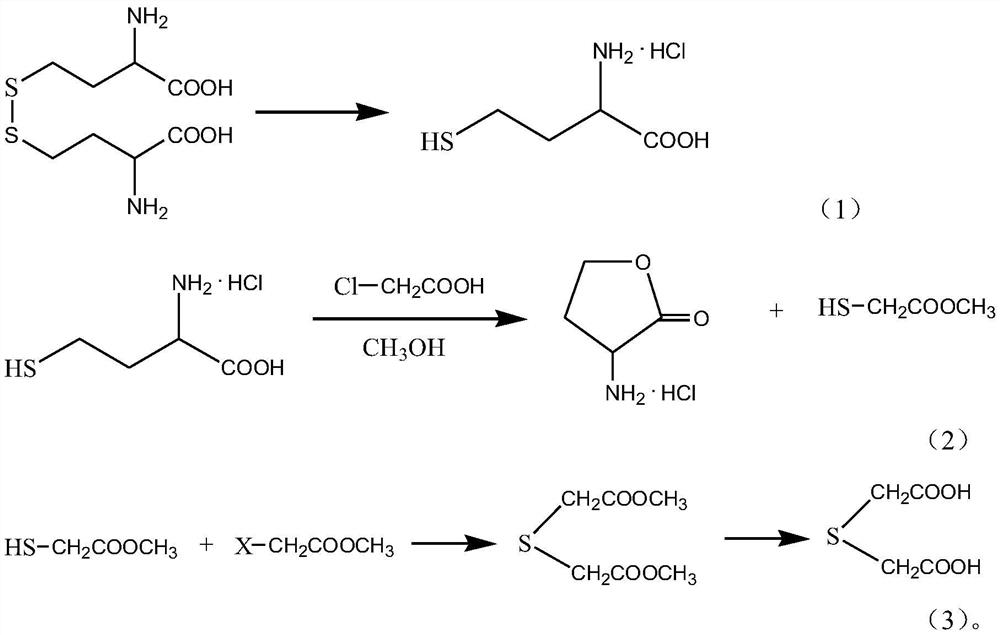
[0025] Step 1. Dissolve homocystine in hydrochloric acid as catholyte, dilute sulfuric acid as anolyte, the anode material of the electrolytic cell is lead-antimony-tin alloy, the cathode material is lead, and a commercially available BPM type bipolar membrane is used between the two poles separated, at an electrolysis temperature of 40°C, a current density of 0.08A / cm 2 , under the condition that the electrolysis time is 10h, the electroreduction reaction is carried out to obtain homocysteine hydrochloride;
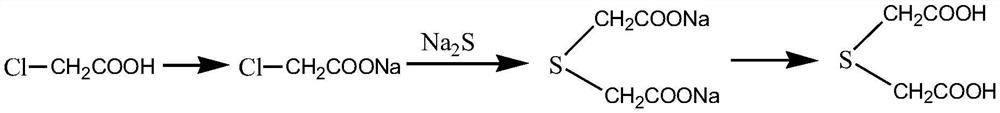
[0026] Step 2. Dissolve the homocysteine hydrochloride and chloroacetic acid in water, heat to 40°C, react for 8 hours, lower the temperature and reduce pressure to recover the solvent water, add methanol to control the temperature at 40°C, and add SO 4 -2 / TiO 2 and thionyl chloride, stirred and reacted for 5h, cooled, filtered out to obtain homoserine lactone hydrochloride, and the filtrate was distilled under reduced pressure to obtain methyl thioglycolate, wher...
example 2
[0029] Step 1, make a film with polystyrene mixed with metal complexes as the intermediate interface catalyst layer, stick the commercially available sulfonic acid type cation exchange membrane, the described intermediate interface catalyst layer, and the commercially available quaternary ammonium salt type anion exchange membrane combined to form a self-made bipolar membrane;
[0030] Step 2, dissolving homocystine in hydrochloric acid as catholyte, dilute sulfuric acid as anolyte, the anode material of the electrolytic cell is lead-antimony-tin alloy, the cathode material is lead, and the two poles are separated by the above-mentioned self-made bipolar membrane , at an electrolysis temperature of 30°C, a current density of 0.09A / cm 2 , under the condition that the electrolysis time is 8 hours, the electroreduction reaction is carried out to obtain homocysteine hydrochloride;
[0031] Step 3: Dissolve the homocysteine hydrochloride and chloroacetic acid in water, heat to...
example 3
[0034] Step 1, using the solution phase inversion method to prepare sulfonic acid type cation exchange membrane and quaternary ammonium salt type anion exchange membrane with finger-like pore structure, dissolve polystyrene particles in organic solvent, add metal complex and mix, use The mixed solution is spun on the surface of the cation-exchange membrane by electrospinning, and then the anion-exchange membrane is glued and fixed on the surface of the cation-exchange membrane spun with polystyrene-metal complex to prepare a self-made bismuth polar membrane.
[0035] Step 2, dissolving homocystine in hydrochloric acid as catholyte, dilute sulfuric acid as anolyte, the anode material of the electrolytic cell is lead-antimony-tin alloy, the cathode material is lead, and the two poles are separated by the above-mentioned self-made bipolar membrane , at an electrolysis temperature of 30°C, a current density of 0.09A / cm 2 , under the condition that the electrolysis time is 8 hours...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com