Kit for quantitatively detecting HBP by using magnetic micro-particle chemiluminiscence, preparation method and detection method thereof
A chemiluminescence, quantitative detection technology, applied in the field of immunological detection, can solve the problems of low sensitivity, complex technical procedures, and non-specific staining problems that have not been completely solved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] A kit for quantitatively detecting human heparin-binding protein (HBP) using magnetic particle chemiluminescence, the kit comprising: HBP calibrator, HBP reagent 1, HBP reagent 2, HBP magnetic separation reagent, and HBP quality control product.
[0068] The preparation method of kit comprises the following contents:
[0069] Step 1. preparing described HBP calibrator comprises: HBP calibrator diluent preparation step and HBP calibrator preparation step:
[0070] The preparation step of described HBP calibrator dilution comprises:
[0071] Add 800mL of purified water and 12.1g of Tris to the container, stir and mix evenly, add 8.5g of sodium chloride, 0.58g of calcium chloride and 1.51g of MIT (methylisothiazolinone), and stir until completely dissolved , adjust the pH value to 8.0-8.5, add 50g of bovine serum albumin into the container, stir until completely dissolved, then adjust the pH value to 8.0-8.5, dilute to 1L with purified water, filter with a 0.2μm filter, ...
Embodiment 2
[0128] A detection method using a kit for the quantitative detection of heparin-binding protein (HBP) using magnetic particle chemiluminescence, comprising the following steps:
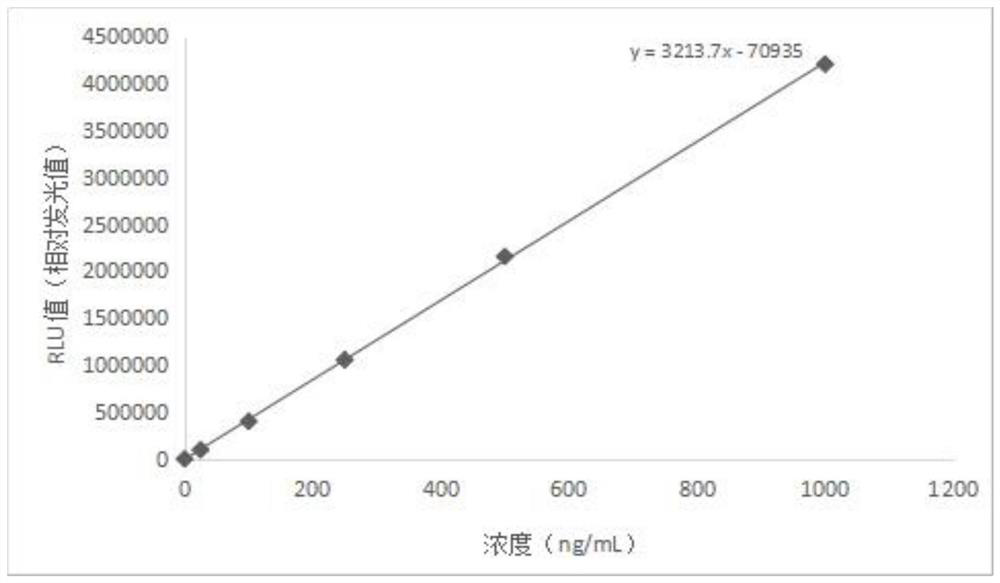
[0129] Step 1. Put the HBP calibrator in the test position of the automatic chemiluminescence immunoassay analyzer, and the analyzer measures the luminescence values at different concentrations of the calibrator, and obtain the fitting of the output luminescence value-concentration value of the automatic chemiluminescence immunoassay analyzer curve, the calibration curve;
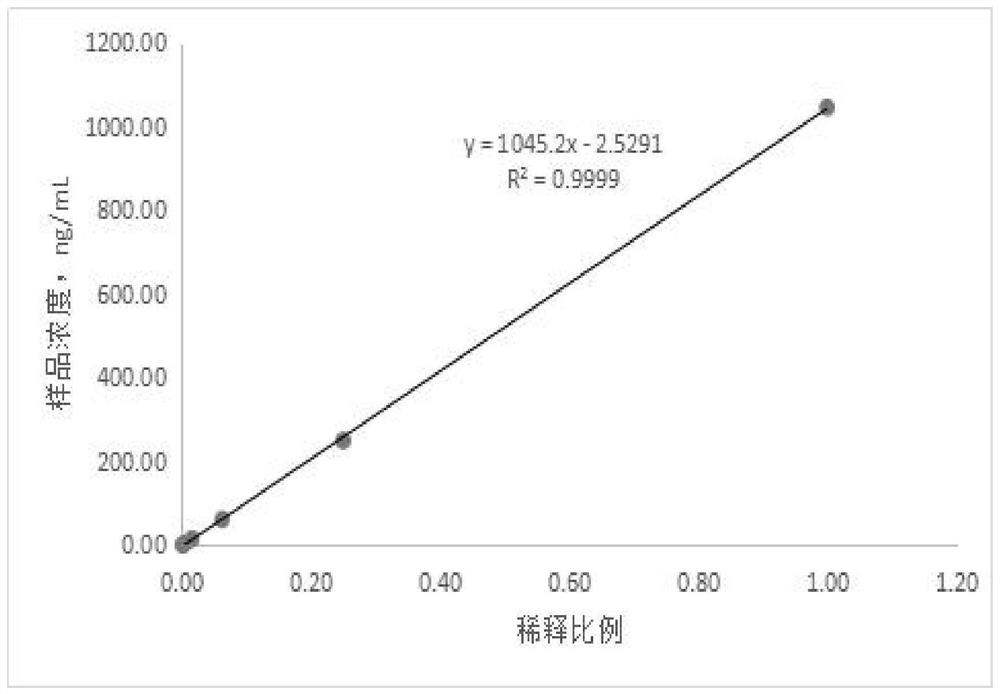
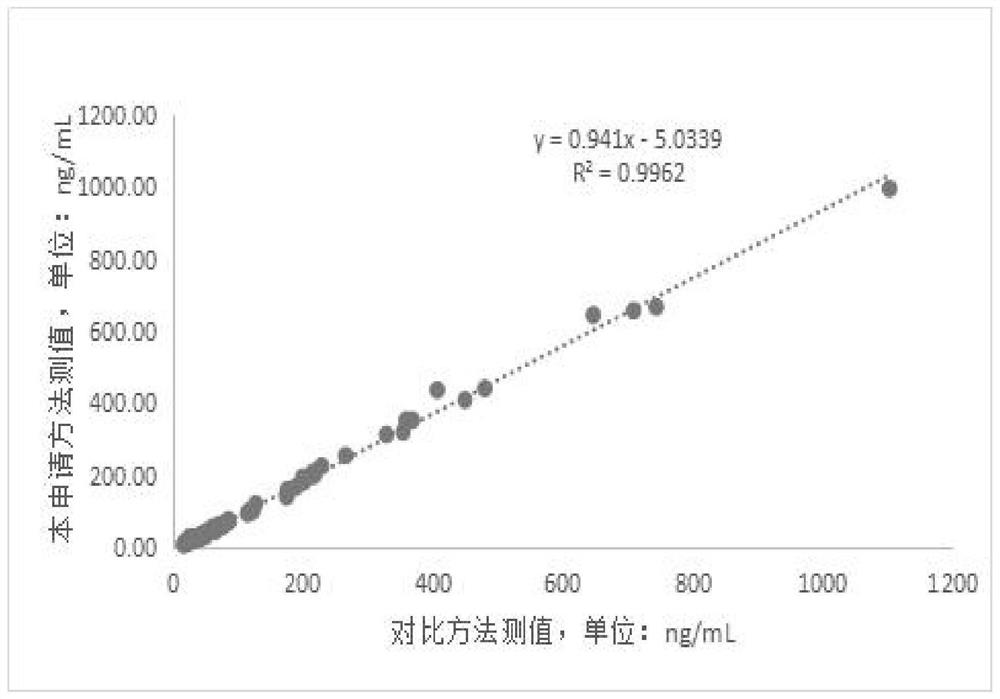
[0130] Step 2. Put the HBP quality control product into the test position of the analyzer, and the analyzer measures the luminescence value under different concentrations of the quality control product, and obtains the test luminescence value of the quality control product output by the automatic chemiluminescence immunoassay analyzer The concentration value of the HBP quality control substance is obtained by fitting the fitting ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com