Triphenylamine-based organic near-infrared fluorescent probe as well as preparation method and application thereof
A fluorescent probe, triphenylamine-based technology, applied in the field of near-infrared fluorescent probes, can solve the problems of no triphenylamine-based near-infrared organic fluorescent probe report, small Stokes shift, short emission wavelength, etc. The effect of high rate, large Stokes shift and longer emission wavelength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A preparation method based on a triphenylamine-based organic near-infrared fluorescent probe, the steps are as follows:
[0058] (1) Synthesis of hept-2,5-diyn-4-ol (1)
[0059] Under a nitrogen atmosphere, put 22.46ml of anhydrous tetrahydrofuran in a 100ml three-necked flask, cool down to -78°C, then add 10ml of n-butyllithium with a syringe, stir for 10 minutes, and then add 0.6005g (15mmol) of propyne in tetrahydrofuran The solution was added dropwise into the reaction flask, and after stirring for 10 minutes, 0.3001 g (5 mmol) of methyl formate was added dropwise into the reaction flask, and the mixture was kept at -78°C for 2 hours, and then the temperature was raised to -40°C. React for 1.5 hours. After the reaction was completed, water was added and the reaction temperature was slowly raised to room temperature. Extracted with diethyl ether (3x300ml), dried over anhydrous magnesium sulfate. The crude product was obtained by distillation under reduced pressure...
experiment example
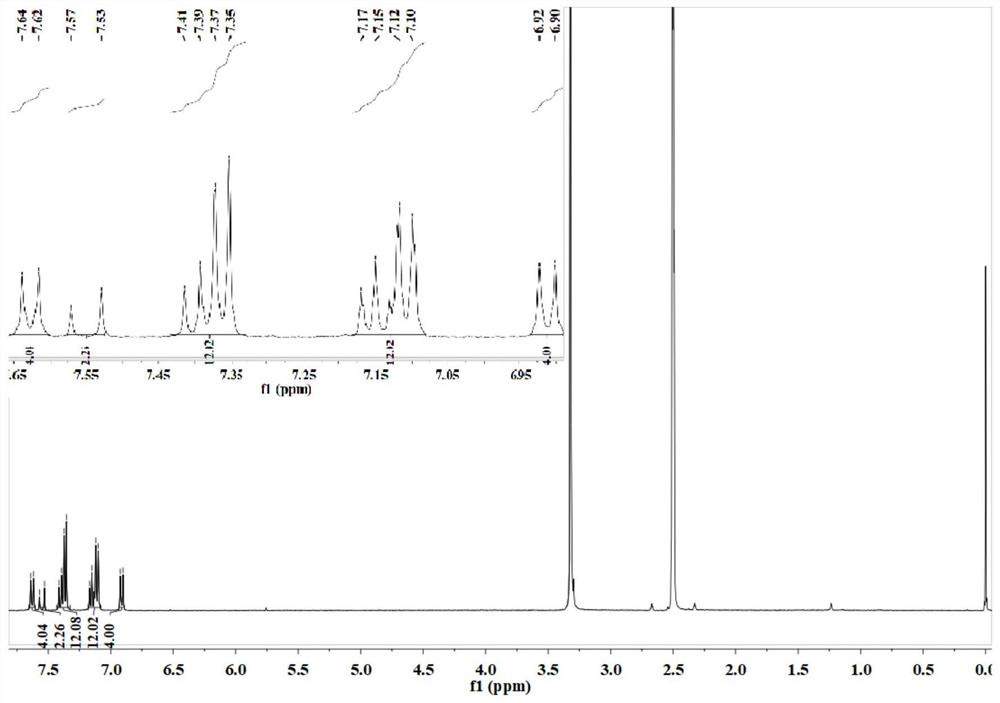
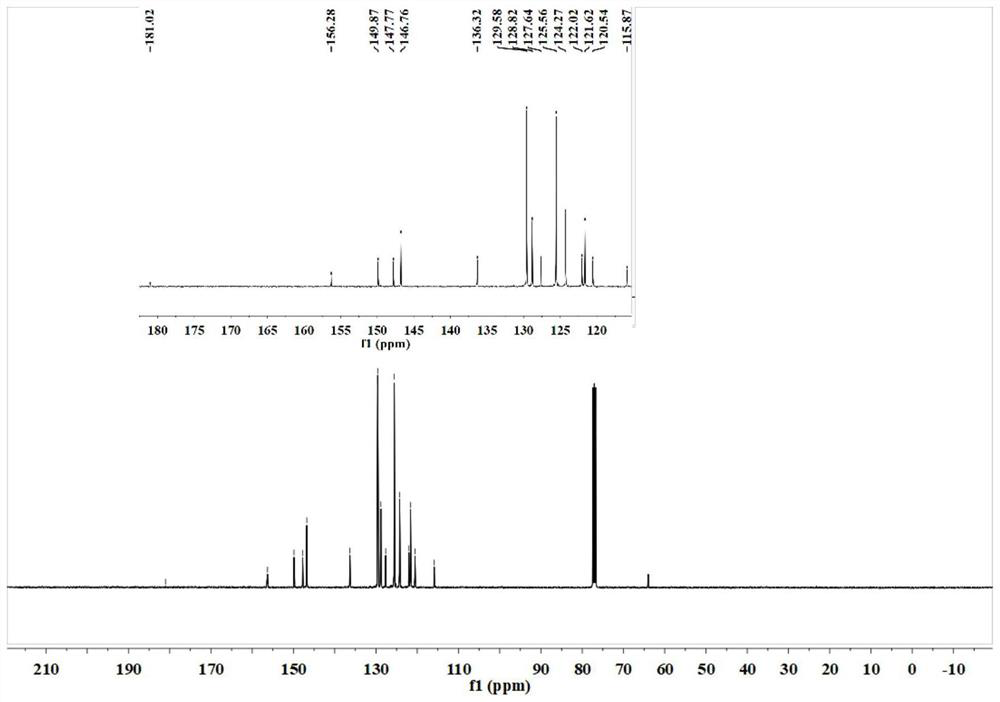
[0083] The organic near-infrared fluorescent probe 2-(2,6-bis((E)-4-(diphenylamino) styryl)-((E)-4-(diphenylamino) styryl)- 4H-thiopyran-4-alkylene) malononitrile for liquid 1 H NMR and liquids 13 C NMR test, test results see respectively Figure 1-Figure 2 shown.
[0084] The organic near-infrared fluorescent probe 2-(2,6-bis((E)-4-(diphenylamino)styryl)-4H-thiopyran-4 prepared above was detected by HITACHI F-7000 fluorescence spectrometer. -Alkylene) malononitrile fluorescence properties are tested, the test results are shown in Figure 4 shown.
[0085] Test Results:
[0086] The excitation wavelength of the organic near-infrared fluorescent probe 2-(2,6-bis((E)-4-(diphenylamino)styryl)-4H-thiopyran-4-alkylene)malononitrile is 510nm, the emission wavelength is 720nm, and the Stokes shift is 210nm. Therefore, the near-infrared fluorescent probe of the present invention has a large Stokes shift and a longer emission wavelength.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com