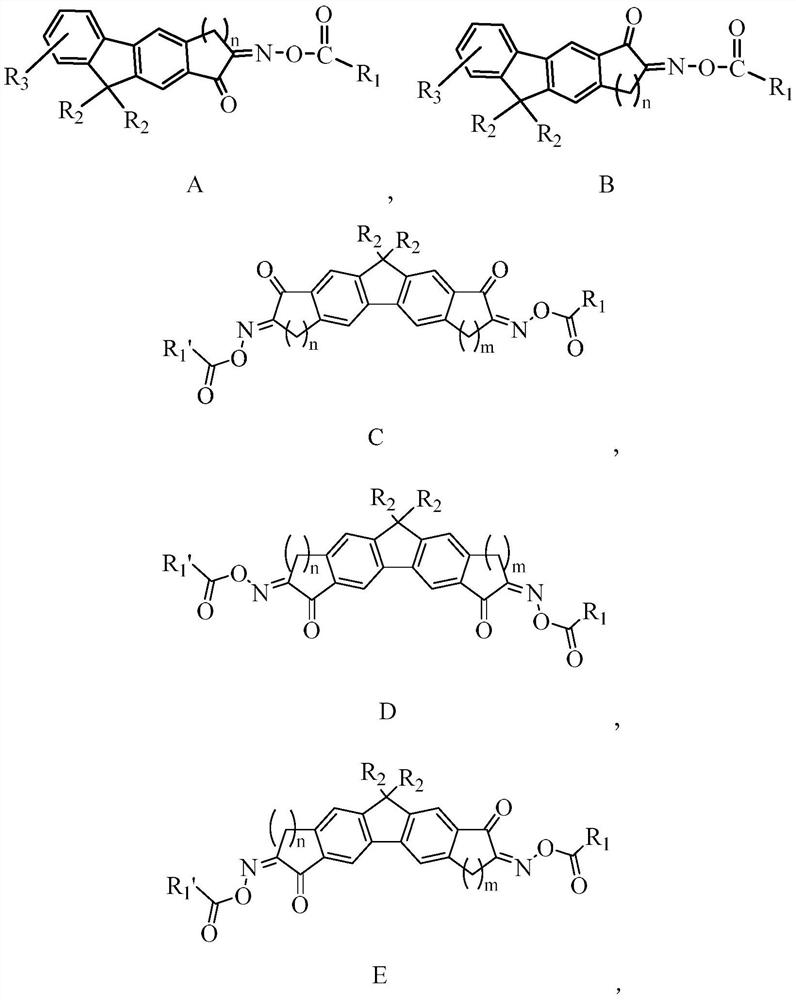
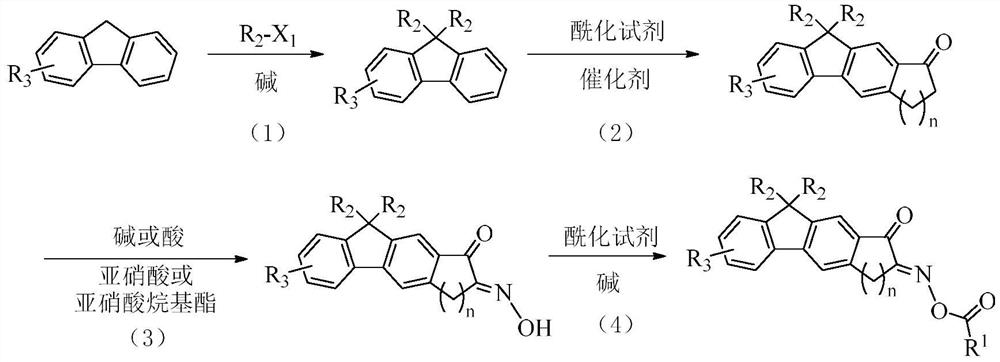
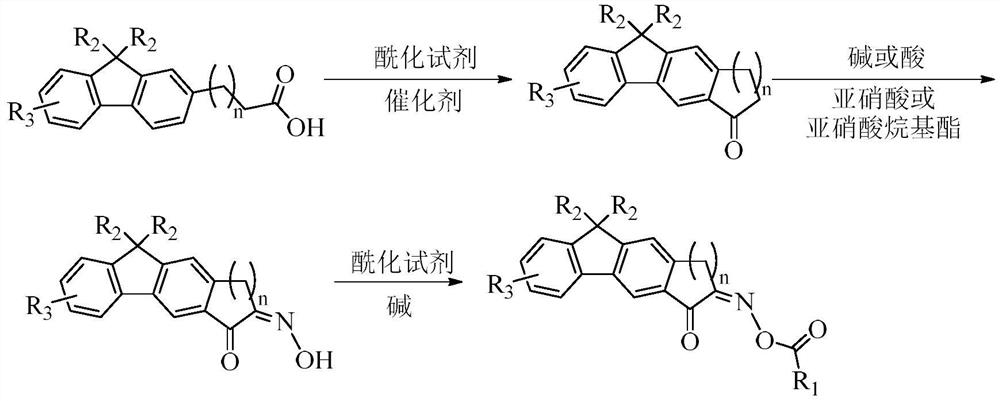
Fluorene oxime ester compound as well as preparation method and application thereof
A technology of ester compounds and compounds, applied in the field of photoinitiators and photosensitive resin compositions, and new fluorene oxime ester compounds, can solve the problems of poor permeability of exposure sources, adhesion of photocurable compositions, alkali resistance and Problems such as decreased transparency, alkali resistance and decreased transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149]Example 1 Preparation of Compound A-1
[0150]
[0151]Preparation of intermediate A-1-1
[0152]
[0153]Add 2-nitro-9H-fluorene (63.3g, 0.3mol), potassium tert-butoxide (77.3g, 0.7mol) and dimethyl sulfoxide (500mL) into a 2000mL round-bottom flask, and slowly add to it under a nitrogen atmosphere Bromobutane (95.6g, 0.7mol) was added dropwise, and after the addition was completed, the reaction was stirred at room temperature. After the reaction is complete, add 1500mL distilled water to quench, extract with ethyl acetate, dry the organic phase with anhydrous magnesium sulfate, distill under reduced pressure to remove the organic solvent, use silica gel column chromatography to purify with ethyl acetate: petroleum ether=1:4, and dry , 70.3 g of intermediate A-1-1 was obtained, with a yield of 72.5%.
[0154]Preparation of intermediate A-1-2
[0155]
[0156]Add Intermediate A-1-1 (32.3g, 0.1mol) and carbon disulfide (1000mL) to a 2000mL round bottom flask, add 2-bromopropionyl bromide (21.6g, 0....
Embodiment 2
[0165]Example 2 Preparation of Compound A-17
[0166]
[0167]The intermediate A-1-3 was synthesized according to the same method as in Example 1 above.
[0168]
[0169]
[0170]Add Intermediate A-1-3 (40.6g, 0.1mol), dichloromethane (1000mL), and triethylamine (20.2g, 0.2mol) to a 2000mL round-bottomed flask. Under the protection of nitrogen, control the temperature at -5 Naphthoyl chloride (38.2g, 0.2mol) was added dropwise at ~0°C, and the reaction was incubated for 5h after the addition was completed. Subsequently, 500 mL of saturated sodium bicarbonate aqueous solution was added to the reaction solution, extracted with dichloromethane, the organic phase was washed with saturated sodium bicarbonate aqueous solution and saturated common salt solution in sequence, dried with anhydrous sodium sulfate, filtered, concentrated, and used on a silica gel column. After purification, 49.9 g of compound A-17 was obtained with a yield of 89.0%.
[0171]The structural characterization data of the product are...
Embodiment 3
[0173]Example 3 Preparation of other compounds
[0174]With reference to the methods of Examples 1 and 2, the corresponding raw materials were replaced to synthesize other compounds of formula A as shown in Table 1 below.
[0175]Table 1
[0176]
[0177]
[0178]
[0179]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com