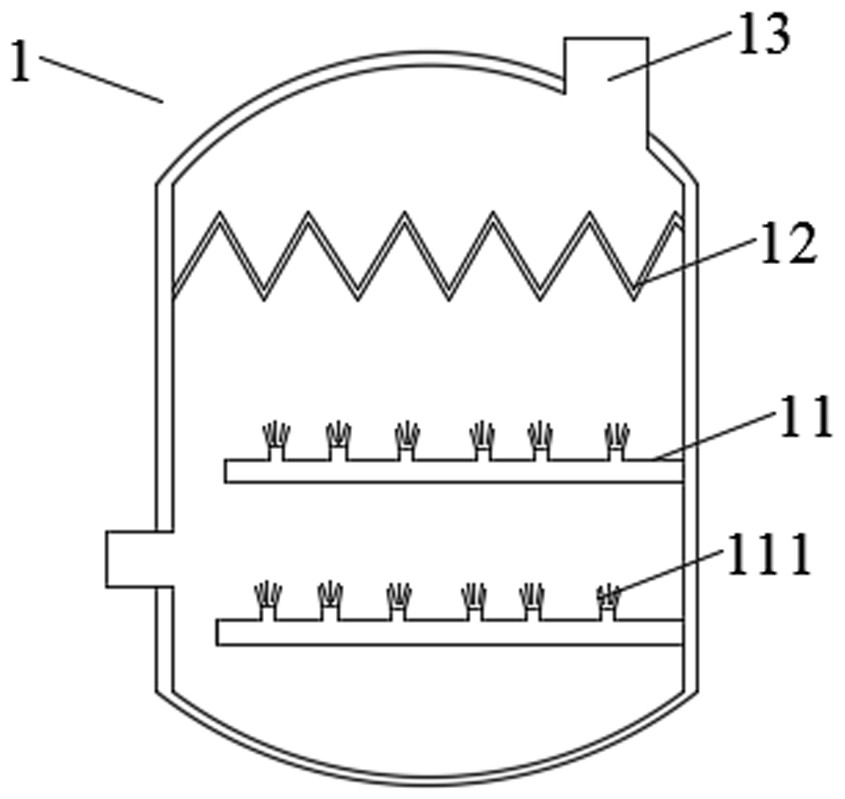
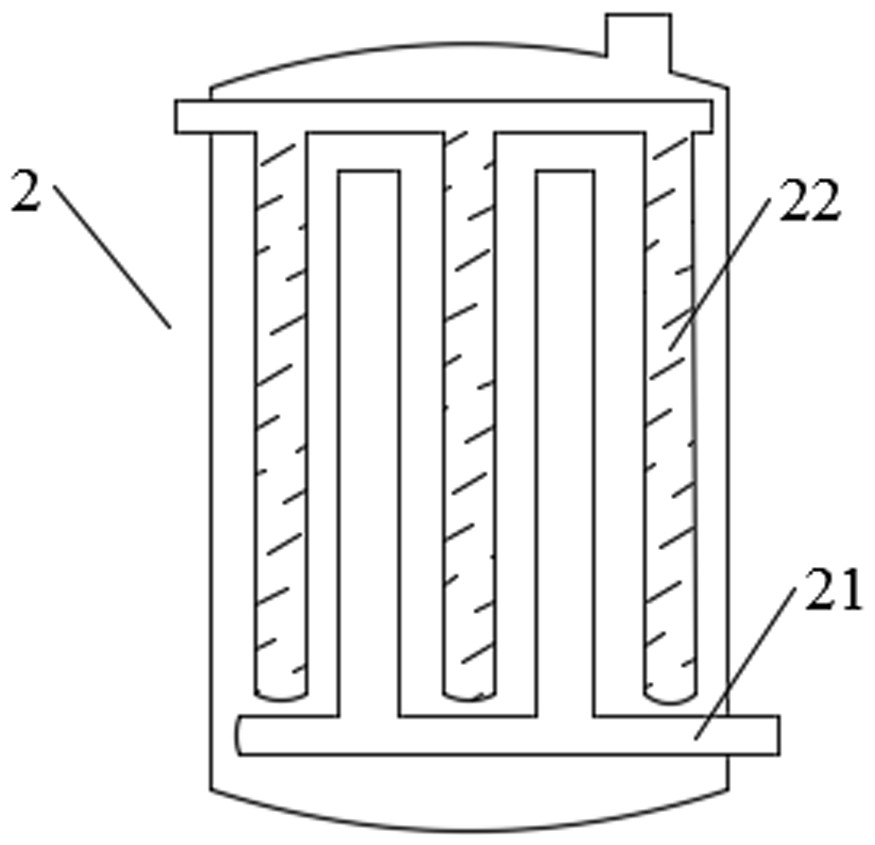
Production method of sodium pyrosulfite with low nitrogen oxide emission
A technology of sodium pyrosulfite and low nitrogen oxides, which is applied in the preparation of alkali metal sulfite, chemical instruments and methods, alkali metal sulfite/sulfite, etc. Pollution, low denitrification efficiency, etc., to achieve the effect of reducing catalytic performance, reducing pollution, and reducing high-temperature areas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A method for producing sodium metabisulfite with low nitrogen oxide emissions, comprising the steps of:
[0034] S1: Send sulfur powder into a low-nitrogen combustion furnace, and carry out spontaneous combustion at 600°C to obtain sulfur dioxide gas.
[0035] S2: Pass the sulfur dioxide gas into the three-stage reactor after dedusting treatment, and react with the soda ash solution to generate a sodium metabisulfite suspension; the gas concentration of the sulfur dioxide is 10%, and the addition amount of the oxygen is Twice the theoretical amount, the temperature at which sulfur dioxide is introduced is 51°C.
[0036] S3: The sodium metabisulfite suspension described in step S2 passes through the secondary reactor and the primary reactor to form sodium metabisulfite crystals, and the finished product can be obtained after the sodium metabisulfite crystals are dried at 150°C.
[0037] S4: After the tail gas generated in step S2 is treated by the lye absorption tower t...
Embodiment 2
[0043] A method for producing sodium metabisulfite with low nitrogen oxide emissions, comprising the steps of:
[0044] S1: Send sulfur powder into a low-nitrogen combustion furnace, and carry out spontaneous combustion at 800°C to obtain sulfur dioxide gas.
[0045] S2: Pass the sulfur dioxide gas into the third-stage reactor after dust removal treatment, and react with the soda ash solution to form a sodium metabisulfite suspension; the gas concentration of the sulfur dioxide is 12%, and the temperature of the sulfur dioxide feeding is 55°C .
[0046]S3: The sodium metabisulfite suspension described in step S2 passes through the secondary reactor and the primary reactor to form sodium metabisulfite crystals, and the finished product can be obtained after the sodium metabisulfite crystals are dried at 170°C.
[0047] S4: After the tail gas generated in step S2 is treated by the lye absorption tower to remove sulfur dioxide, it is compressed by a pressurizing device and passe...
Embodiment 3
[0050] A method for producing sodium metabisulfite with low nitrogen oxide emissions, comprising the steps of:
[0051] S1: Send sulfur powder into a low-nitrogen combustion furnace, and carry out spontaneous combustion at 1100°C to obtain sulfur dioxide gas.
[0052] S2: Pass the sulfur dioxide gas into the third-stage reactor after dedusting treatment, and react with the soda ash solution to form a sodium metabisulfite suspension; the gas concentration of the sulfur dioxide is 15%, and the temperature of the sulfur dioxide feeding is 60°C .
[0053] S3: The sodium metabisulfite suspension described in step S2 passes through the secondary reactor and the primary reactor to form sodium metabisulfite crystals, and the finished product can be obtained after the sodium metabisulfite crystals are dried at 180°C.
[0054] S4: After the tail gas generated in step S2 is treated by the lye absorption tower to remove sulfur dioxide, it is compressed by a pressurizing device and passed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com