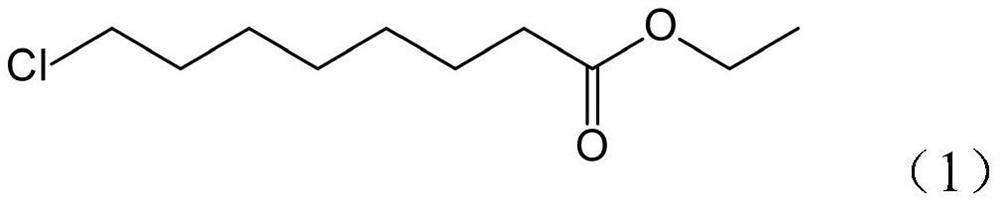
Synthesis method of 8-chloro ethyl caprylate
A technology of ethyl chlorooctanoate and a synthesis method, which is applied in the preparation of carboxylate, chemical instruments and methods, preparation of carboxylate, etc., can solve the problems of low yield, many side reactions, unsuitable for industrial production and the like, and achieves purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
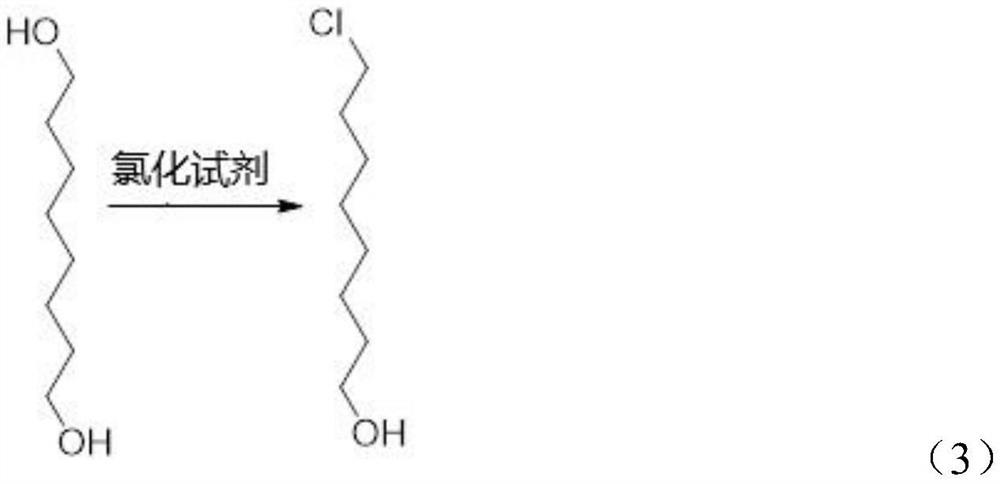
[0028] According to the synthetic method of the 8-chlorooctanoic acid ethyl ester of the embodiment of the present invention, comprise the steps:
[0029] Step S1, chlorinating 1,8-octanediol with a chlorination reagent in an aromatic hydrocarbon solvent to obtain 8-chloro-1-octanol.
[0030] Specifically, its chemical reaction formula is shown in the following formula (3):
[0031]
[0032] According to the synthesis method of ethyl 8-chlorooctanoate in the embodiment of the present invention, 1,8-octanediol is used as a raw material, and the raw material is easy to obtain and low in price.
[0033] In addition, in the step S1, the aromatic hydrocarbon solvent is one or more selected from benzene, toluene, and xylene, and the chlorination reagent is selected from phosphorus trichloride, hydrochloric acid, thionyl chloride, One or more of anhydrous zinc chloride and chlorine gas. The raw material 1,8-octanediol used in the present invention can be well dissolved in these ...
Embodiment 1
[0057] Pour the raw material 1,8-octanediol (80g, 1.0eq), 30% hydrochloric acid solution (599g 9.0eq) and solvent toluene (200mL) into the reaction bottle, start stirring, raise the temperature for reflux reaction, control the reaction temperature at 102-115 Celsius, and continued to react for 10 h after heating up to reflux state.
[0058] After the completion of the reaction detected by central control GC, the water layer was separated, the organic layer was washed with water and dried, and the solvent was removed under reduced pressure to obtain the crude compound. The crude product was purified by vacuum distillation to obtain 70.2 g of the compound 8-chloro-1-octanol, the fraction temperature was 56-63 degrees Celsius, and the yield was 78%.
[0059] Pour the compound 8-chloro-1-octanol (60g 1.0eq) and the solvent dichloromethane (270mL) into the reaction flask, start stirring, add sodium hypochlorite (81.3g, 3.0eq) in batches, control the reaction temperature 45-55 degr...
Embodiment 2
[0063] Pour the raw material 1,8-octanediol (2kg, 1.0eq), 30% hydrochloric acid solution (13.3kg 8.0eq) and solvent toluene (5L) into the reaction bottle, start stirring, heat up for reflux reaction, control the reaction temperature 103~ 113 degrees Celsius, after heating up to the reflux state, the reaction was continued for 12 hours.
[0064] The central control GC detects that the reaction is complete, and the water layer is separated. The organic layer is washed and dried, and the solvent is removed under reduced pressure to obtain the crude product of compound 8-chloro-1-octanol. The crude product is purified by distillation under reduced pressure to obtain the compound 8-chloro-1-octanol Alcohol 1824g, distillate temperature 56~63 degrees centigrade, yield 81%.
[0065] Pour the compound 8-chloro-1-octanol (1824g 1.0eq) and the solvent dichloromethane (8200mL) into the reaction flask, start stirring, put in the raw material sodium hypochlorite (3309g 4.0eq) in batches fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com