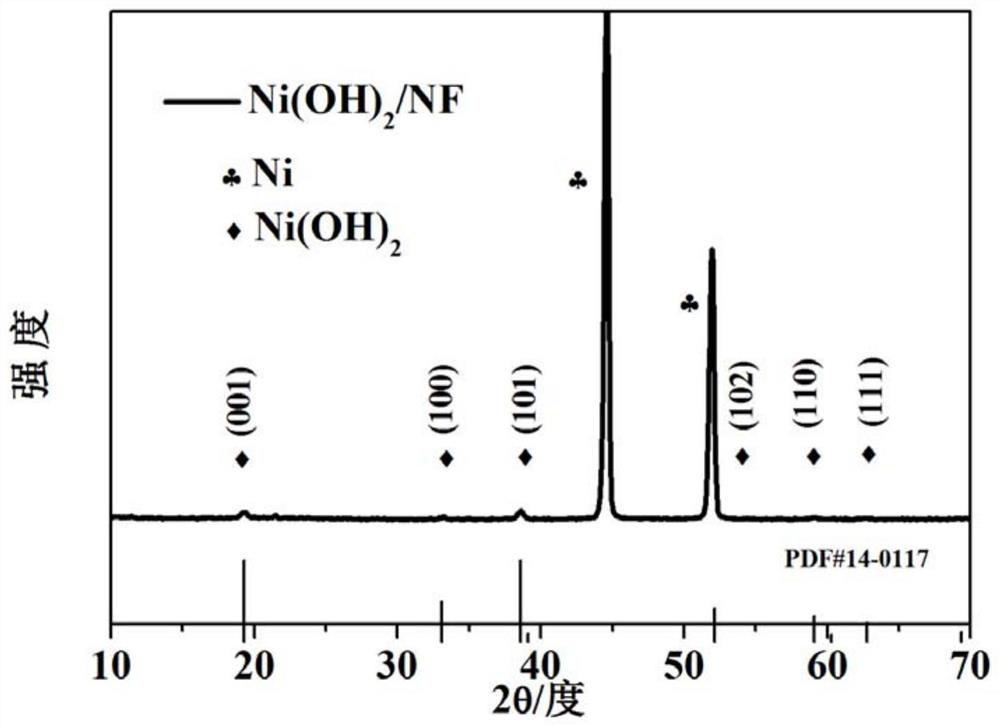
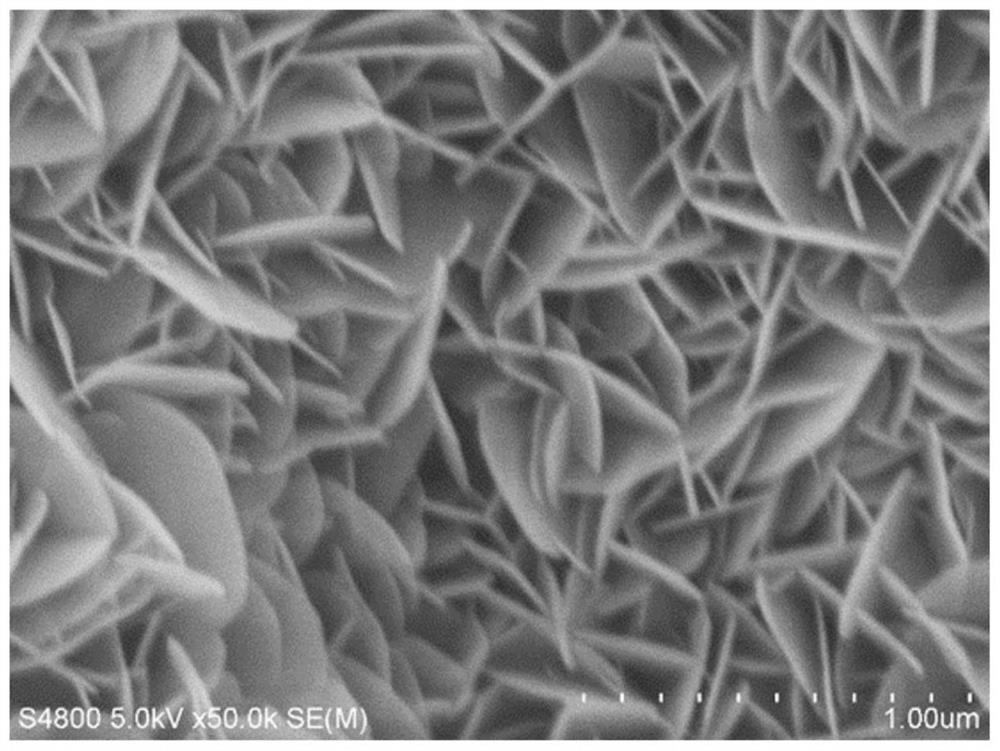
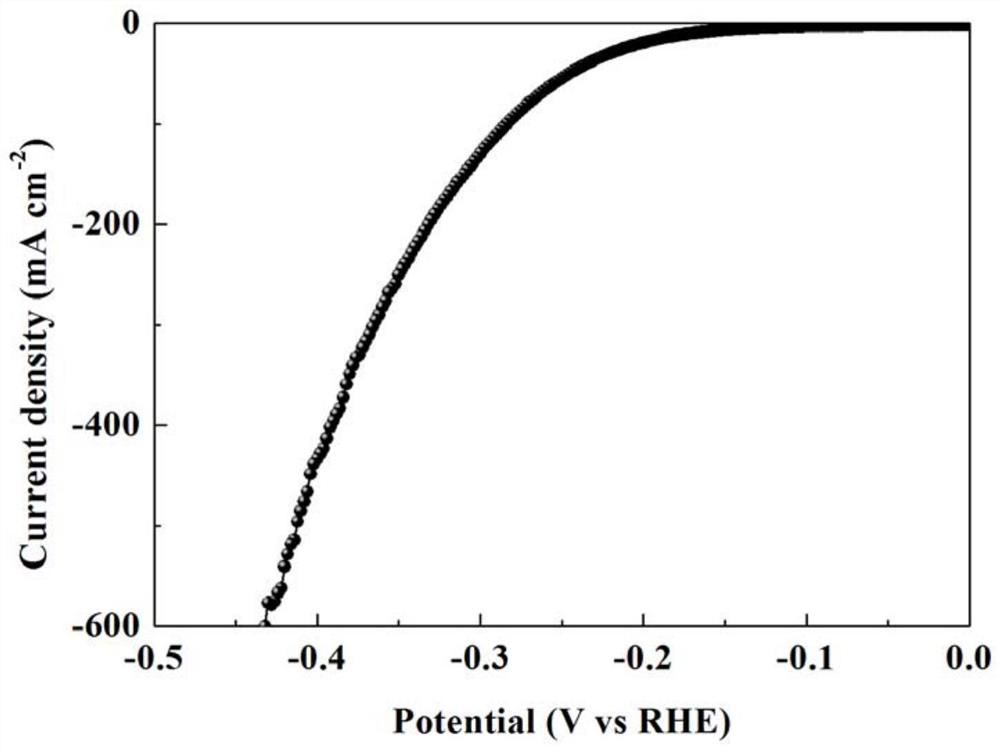
Chromium-aluminum co-doped nickel-based hydroxide self-supporting electrode for fully decomposing water and preparation method thereof
A self-supporting electrode, hydroxide technology, applied in electrodes, electrolysis process, electrolysis components, etc., can solve the problem of carbon dioxide emission and environmental pollution, high potential barrier and slow reaction rate of electrolyzed water, difficult to promote and use on a large scale, etc. problem, to achieve the effect of improving catalytic activity and stability, multiple reactive active sites, and convenient open area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation method of the chromium-aluminum co-doped nickel-based hydroxide self-supporting electrode for total water splitting of the present invention, the specific steps include:
[0026] Step 1: Pretreat the nickel foam. Cut the nickel foam into 1cm×5cm blocks, ultrasonically clean it in acetone solution for 10min, then pour it into the prepared 3mol / L hydrochloric acid solution for ultrasonic cleaning for 5min, and finally rinse it alternately with absolute ethanol and ultrapure water 3 times, and then vacuum-dried at 25°C for 12 hours before use;
[0027] Step 2: Mix 170mg of nickel nitrate hexahydrate, 55mg of chromium nitrate nonahydrate, 40mg of aluminum chloride hexahydrate, 125mg of urea, 1.5mL of dimethylformamide and 0.25g of polyvinylpyrrolidone, and add 20ml ultrapure water, magnetically stirred for 20min, and stirred evenly to obtain solution A;
[0028] Step 3: Soak the spare nickel foam obtained in step 1 in the solution A obtained in step 2, tra...
Embodiment 2
[0035] The preparation method of the chromium-aluminum co-doped nickel-based hydroxide self-supporting electrode for total water splitting of the present invention, the specific steps include:
[0036] Step 1: Pretreat the nickel foam. Cut the nickel foam into 1cm×5cm blocks, ultrasonically clean it in acetone solution for 15min, then pour it into the prepared 1mol / L hydrochloric acid solution for ultrasonic cleaning for 10min, and finally rinse it alternately with absolute ethanol and ultrapure water 2 times, and then vacuum-dried at 35°C for 10 hours before use;
[0037] Step 2: Mix 180mg of nickel nitrate hexahydrate, 65mg of chromium nitrate nonahydrate, 30mg of aluminum chloride hexahydrate, 115mg of urea, 1.6mL of dimethylformamide and 0.26g of polyvinylpyrrolidone, and add 21ml ultrapure water, magnetically stirred for 21min, and stirred evenly to obtain solution A;
[0038] Step 3: Soak the spare nickel foam obtained in step 1 in the solution A obtained in step 2, tr...
Embodiment 3
[0041] The preparation method of the chromium-aluminum co-doped nickel-based hydroxide self-supporting electrode for total water splitting of the present invention, the specific steps include:
[0042]Step 1: Pretreat the nickel foam. Cut the nickel foam into 1cm×5cm blocks, ultrasonically clean it in acetone solution for 11min, then pour it into the prepared 2mol / L hydrochloric acid solution for ultrasonic cleaning for 8min, and finally rinse it alternately with absolute ethanol and ultrapure water 3 times, and then vacuum-dried at 30°C for 11 hours before use;
[0043] Step 2: Mix 175mg of nickel nitrate hexahydrate, 60mg of chromium nitrate nonahydrate, 35mg of aluminum chloride hexahydrate, 120mg of urea, 1.7mL of dimethylformamide and 0.27g of polyvinylpyrrolidone, and add 22ml ultrapure water, magnetically stirred for 22min, and stirred evenly to obtain solution A;
[0044] Step 3: Soak the spare nickel foam obtained in step 1 in the solution A obtained in step 2, tran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com