Preparation method of lithium ion battery positive electrode material
A technology for lithium-ion batteries and cathode materials, which is applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of small ion or electron diffusion coefficient, poor cycle stability of transition metal sulfides, and rapid capacity decay, and achieve enhanced Electrochemical performance, increase electrochemical performance, good effect of electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A preparation method of lithium ion battery cathode material, comprising the steps of:
[0025] (1) Add 70mg of ferric chloride hexahydrate, 5mg of nickel chloride hexahydrate and 5mg of carbon nanotubes to 10ml of a mixed solvent composed of water and DMF (N,N-dimethylformamide) at a volume ratio of 1:4 Then add 1.5ml of 0.1M sodium sulfide aqueous solution, heat and reflux at 100°C for 20h, then transfer the obtained mixture into a hydrothermal kettle and conduct a hydrothermal reaction at 200°C for 8h to obtain a hydrothermal reaction product;
[0026] (2) The hydrothermal reaction product is subjected to microwave plasma treatment for 10 minutes (the microwave frequency is 2.45 GHz, the power is 150 W, and the vacuum degree is 5 Pa).
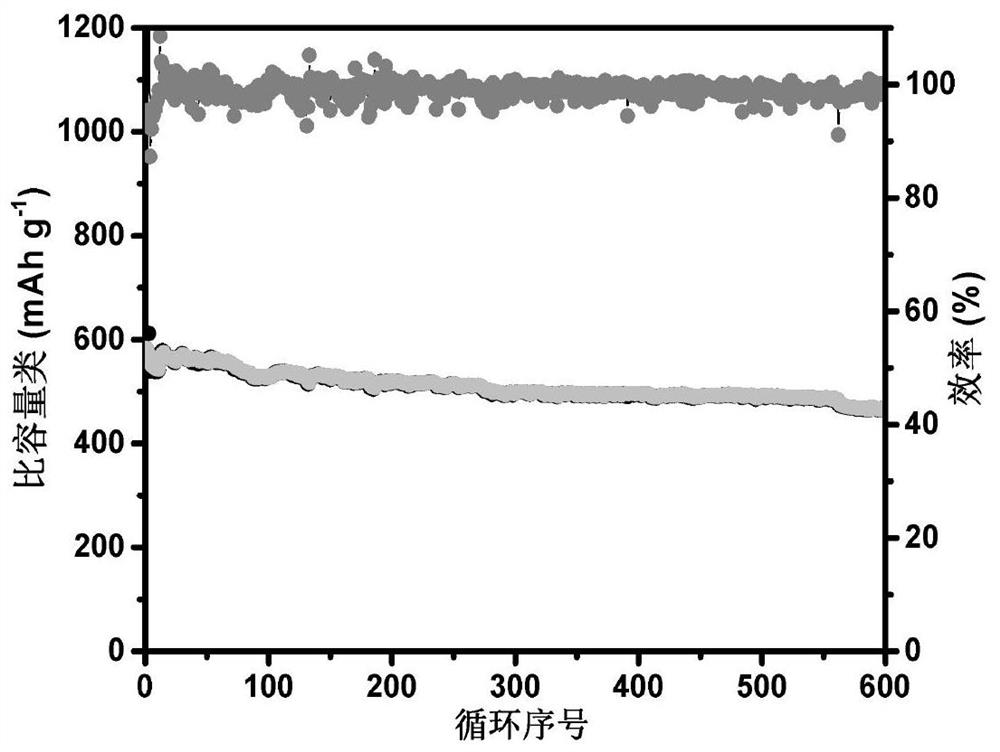
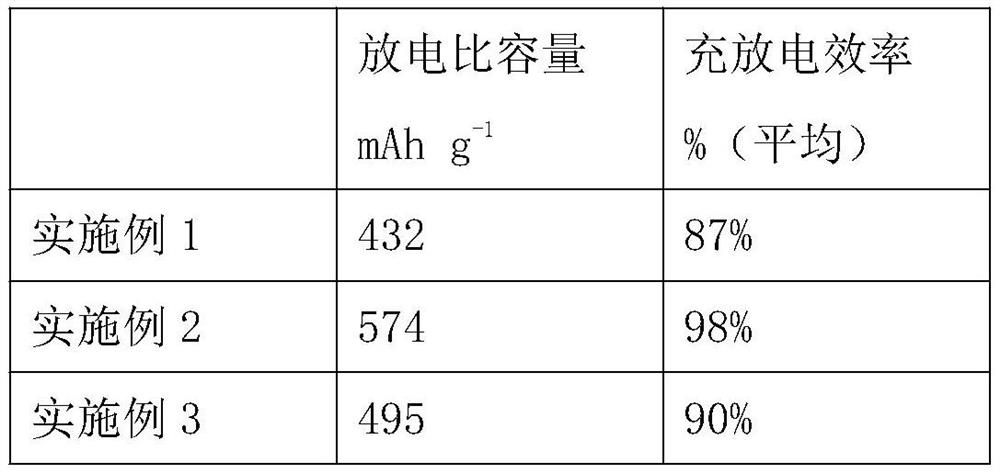
[0027] Through the BET test, the specific surface area of the material is 72.5m 2 / g.
Embodiment 2
[0029] A preparation method of lithium ion battery cathode material, comprising the steps of:
[0030] (1) Add 70 mg of ferric chloride hexahydrate, 10 mg of nickel chloride hexahydrate and 7 mg of carbon nanotubes to 12.5 ml of a mixture of water and DMF (N,N-dimethylformamide) at a volume ratio of 1:4 into the solvent, then add 2ml of 0.1M sodium sulfide aqueous solution, heat and reflux at 100°C for 16h, then transfer the obtained mixture into a hydrothermal kettle for hydrothermal reaction at 200°C for 10h, to obtain a hydrothermal reaction product;
[0031] (2) The hydrothermal reaction product is subjected to microwave plasma treatment for 20 minutes (the microwave frequency is 2.45 GHz, the power is 150 W, and the vacuum degree is 5 Pa).
[0032] Through the BET test, the specific surface area of the material is 211.3m 2 / g.
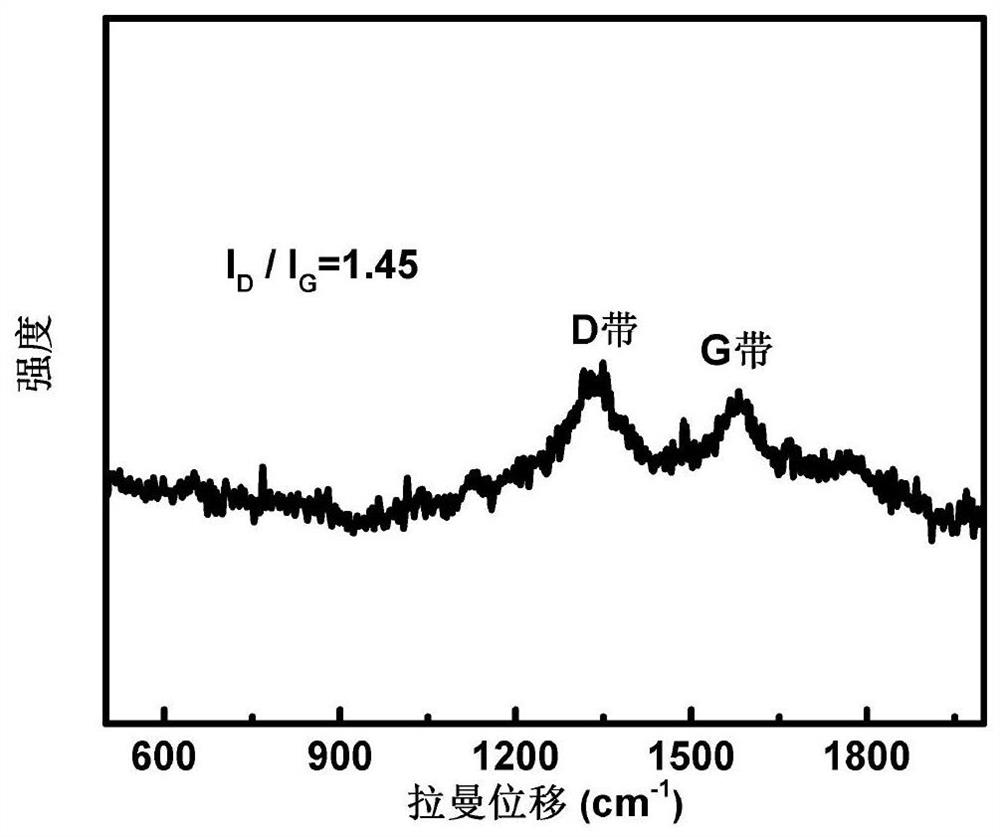
[0033] The Raman shift spectrogram of the lithium-ion battery positive electrode material that the present embodiment makes is shown in figu...
Embodiment 3
[0035] A preparation method of lithium ion battery cathode material, comprising the steps of:
[0036] (1) Add 73mg of ferric chloride hexahydrate, 14mg of nickel chloride hexahydrate and 8mg of carbon nanotubes to 12.5ml of a mixture of water and DMF (N,N-dimethylformamide) at a volume ratio of 1:4 into the solvent, then add 2ml of 0.1M aqueous sodium sulfide solution, heat and reflux at 100°C for 12h, then transfer the obtained mixture into a hydrothermal kettle for hydrothermal reaction at 220°C for 12h to obtain a hydrothermal reaction product;
[0037] (2) The hydrothermal reaction product is subjected to microwave plasma treatment for 20 minutes (the microwave frequency is 2.45 GHz, the power is 150 W, and the vacuum degree is 5 Pa).
[0038] Through the BET test, the specific surface area of the material is 132.6m 2 / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com