Synthesis method of 2,4-diaminobutyric acid derivative
A diaminobutyric acid and synthesis method technology, applied in the field of synthesis of 2,4-diaminobutyric acid derivatives, can solve the problems of troublesome post-processing and cumbersome steps, and achieve easy separation and purification, mild reaction conditions, and convenience for later stages Amplify the effect of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
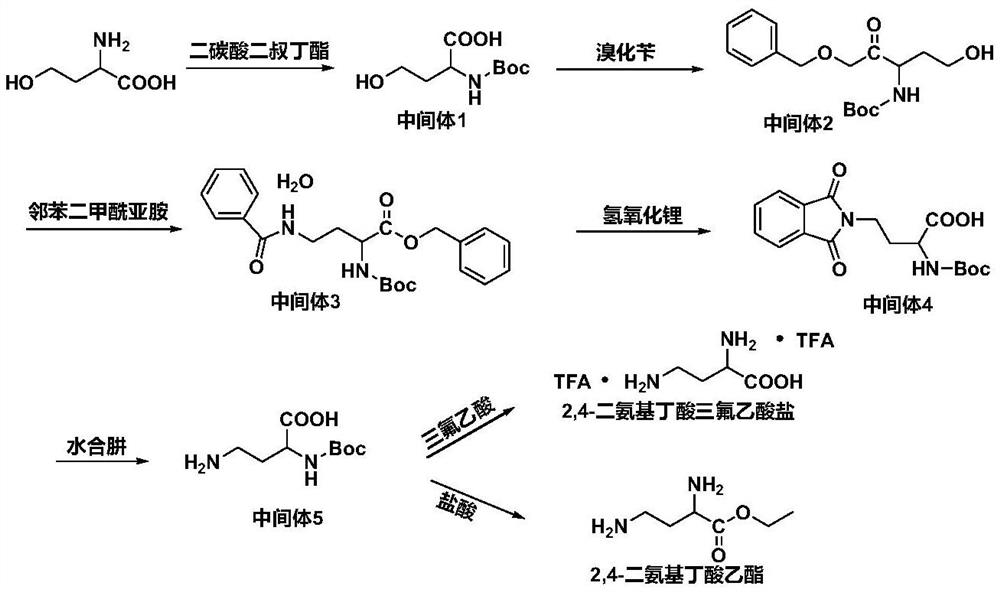
[0036] The synthetic method of 2,4-diaminobutyric acid derivative comprises the following steps:
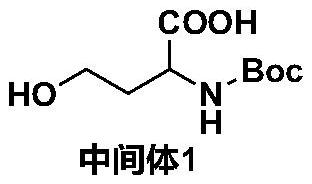
[0037] a. Dissolve homoserine in a mixed solvent, add an aqueous alkali solution, and drop di-tert-butyl dicarbonate solution dissolved in tetrahydrofuran at -5 to 5°C. After the addition, react at room temperature for 12 to 48 hours; the reaction is over Finally, the concentrated reaction solution was extracted with petroleum ether, the pH of the reaction solution was adjusted to 1-5 at -5-5°C, extracted with ethyl acetate, the extract was washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and the organic layer was concentrated , column chromatography separation to obtain intermediate 1; the molar ratio of homoserine and di-tert-butyl dicarbonate is 1:1~1:2;
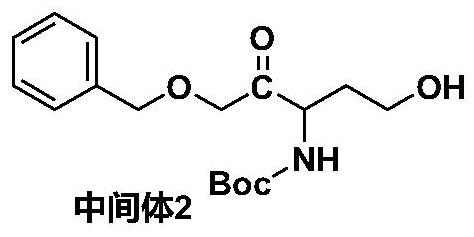
[0038] b. Mix intermediate 1 and triethylamine in an organic solvent, add benzyl bromide dropwise under reflux, and react for 4 to 8 hours after the dropwise addition; add water to the rea...
Embodiment 1
[0057] Embodiment 1 synthesis condition screening experiment
[0058] 1. Screening of ester hydrolysis conditions during the preparation of intermediate 4
[0059] The ester hydrolysis reagent that the present invention chooses initially is LiOH, NaOH, KOH, K 2 CO 3 and Na 2 CO 3 .
[0060] Intermediate 3 was mixed with the above-mentioned different ester hydrolysis reagents in 200 mL of methanol: water = 4: 1 (V / V) mixed solvent, and reacted at room temperature for 4 h. The reaction solution was spin-dried, adjusted to pH 1 with concentrated hydrochloric acid in an ice bath, extracted five times with dichloromethane (50 mL), washed once with saturated sodium chloride solution (50 mL), and dried over anhydrous sodium sulfate. Wherein, the molar ratio of intermediate 3 to each ester hydrolysis reagent is 1:20. The specific experimental results are shown in Table 1.
[0061] Table 1 Yield using different ester hydrolysis reagents
[0062] LiOH NaOH KOH K ...
Embodiment 2
[0071] Synthesis of Embodiment 2 Intermediate 1 (N-tert-butoxycarbonyl-homoserine)
[0072]
[0073] Dissolve homoserine (83.96mmol, 10.00g) in 250ml of ethanol: water = 2:1 (V / V) mixed system, add 84ml of 1N NaOH aqueous solution, stir the solution until it is clear and transparent, and place it in an ice bath A solution of di-tert-butyl dicarbonate (92.36 mmol, 20.16 g) dissolved in 84 ml of tetrahydrofuran was added dropwise, and after the addition was completed, the mixture was reacted at room temperature for 24 h.
[0074] The concentrated reaction solution was extracted 3 times with petroleum ether (100mL) to remove some impurities, the pH of the reaction solution was adjusted to 2 under ice bath, extracted 5 times with ethyl acetate (100mL), and the extract was washed once with saturated sodium chloride solution (30mL) , dried over anhydrous sodium sulfate. The organic layer was concentrated and separated by column chromatography (dichloromethane:methanol=2:1) to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com