Preparation method of ibuprofen impurity B
An impurity and compound technology, applied in the field of preparation of pharmaceutical impurity standard products, can solve the problems of difficult reaction and cannot be satisfied, and achieve the effects of short preparation period, convenient operation and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of intermediate formula 2 compound
[0037] Add the compound of formula 1 (1.78 g, 10 mmol, 1.0 eq) into the three-neck flask, Pd(PPh 3 ) 4 (347mg, 0.3mmol, 0.03eq), Cu 2 O (43 mg, 0.3 mmol, 0.03 eq), K 2 CO 3 (2.07 g, 15 mmol, 1.5 eq) and toluene (20 mL), vacuumize, replace with argon 3 times, then stir and heat up to 80°C for 18 hours, TLC monitors 1 reaction is complete, cool down to room temperature, filter with diatomaceous earth , the filtrate was concentrated to remove the solvent, purified by silica gel column chromatography, the elution condition was: petroleum ether: ethyl acetate = 20:1, and 1.92 g of the intermediate compound of formula 2 was obtained, with a yield of 87.3%.
Embodiment 2
[0038] Embodiment 2: the preparation of intermediate formula 3 compound
[0039] Under nitrogen protection, add intermediate 2 compound (1.76g, 8 mmol, 1.0 eq) dissolved in dry tetrahydrofuran (15 mL) into the three-necked flask, then stir and cool to -78°C, slowly add 1.0 M bistrimethyl A tetrahydrofuran solution of lithium silylamide (8.8 mL, 8.8 mmol, 1.1 eq) was added dropwise and continued to stir for 30 minutes, then CH dissolved in dry THF (2 mL) was added dropwise 3 I (0.5 mL, 8 mmol, 1.0eq), continue stirring for 1 hour after the dropwise addition, then return to room temperature and continue stirring for 2 hours, then lower to 0°C, slowly add saturated NH 4 Cl solution (20 mL), then extracted with EA (20 mL×3), the organic phases were combined, washed with saturated brine, separated, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, concentrate to remove the solvent, and purify by silica gel column chromatography, the elution condition is: petroleum et...
Embodiment 3
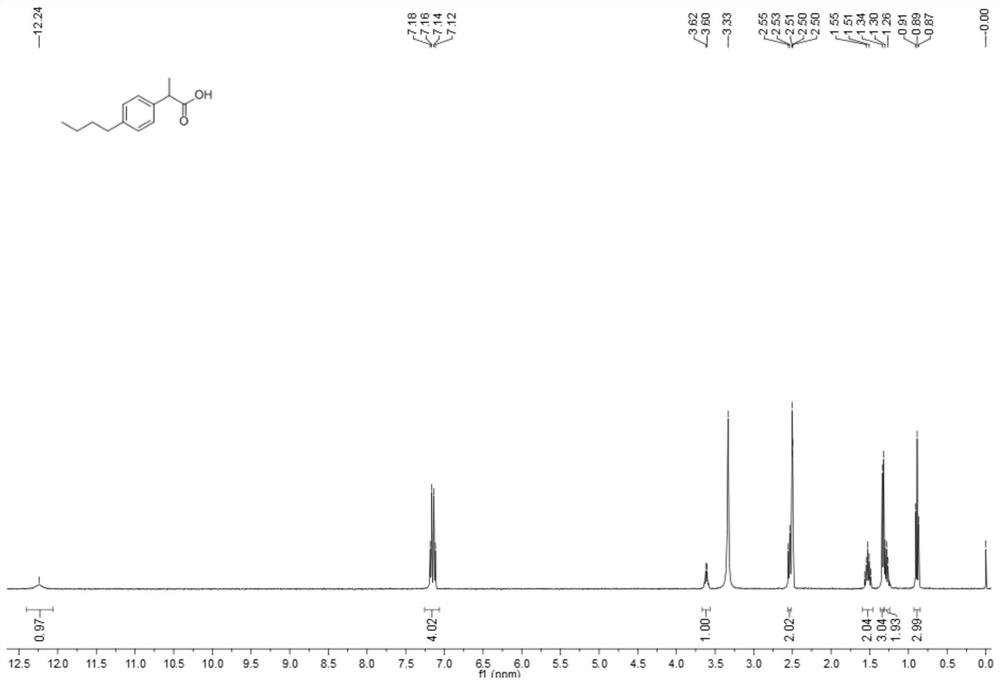
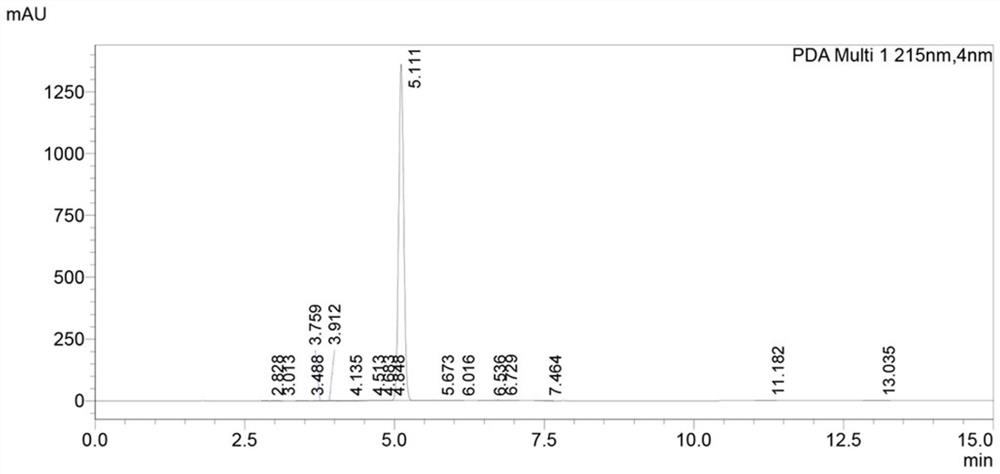
[0040] Embodiment 3: the preparation of ibuprofen impurity B
[0041] Add intermediate 3 compound (1.64 g, 7 mmol, 1.0 eq) and ethanol (15 mL) into a 100 mL single-necked bottle, and add sodium hydroxide (0.56 g, 14 mmol, 2.0 eq) solution, the reaction was continued for 3 hours after the dropwise addition, and the reaction was completed by TLC monitoring 3, and the pH was adjusted to 3~4 with 1N HCl. Then it was extracted with EA (20 mL×3), the organic phases were combined, washed with saturated brine, separated, and the organic phase was washed with anhydrous Na 2 SO 4 Dry, concentrate to remove the solvent, and purify by silica gel column chromatography. The elution condition is: dichloromethane: methanol: acetic acid = 50:1:0.1 to obtain 1.38 g of ibuprofen impurity B with a yield of 95.5%.
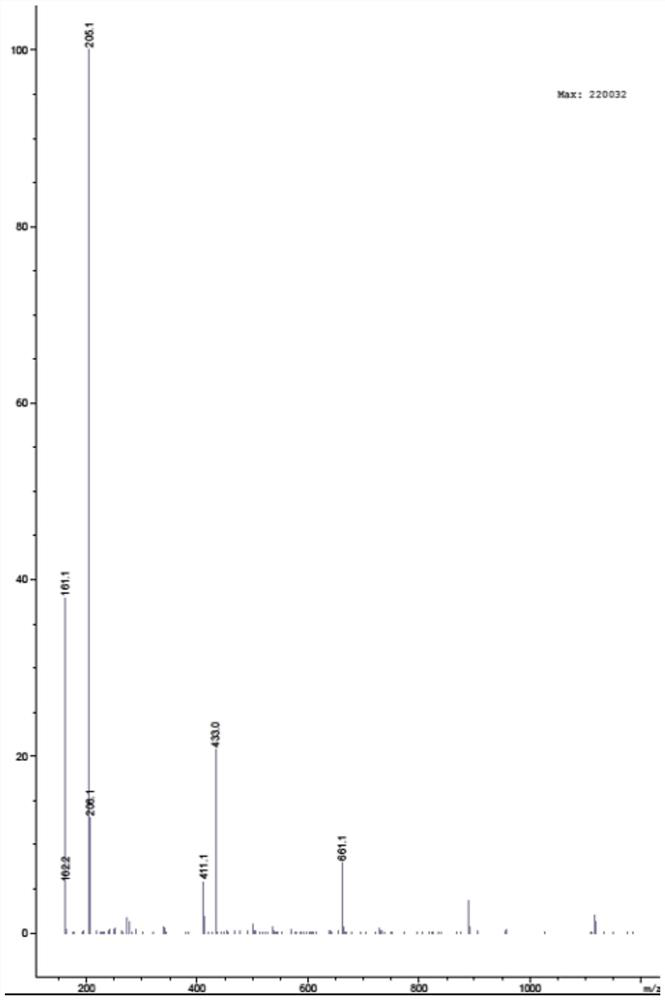
[0042] Molecular formula of ibuprofen impurity B: C 13 h 18 o 2 ;
[0043] Molecular weight of ibuprofen impurity B: 206.28.
[0044] Adopt mass spectrometer to analyze syntheti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com