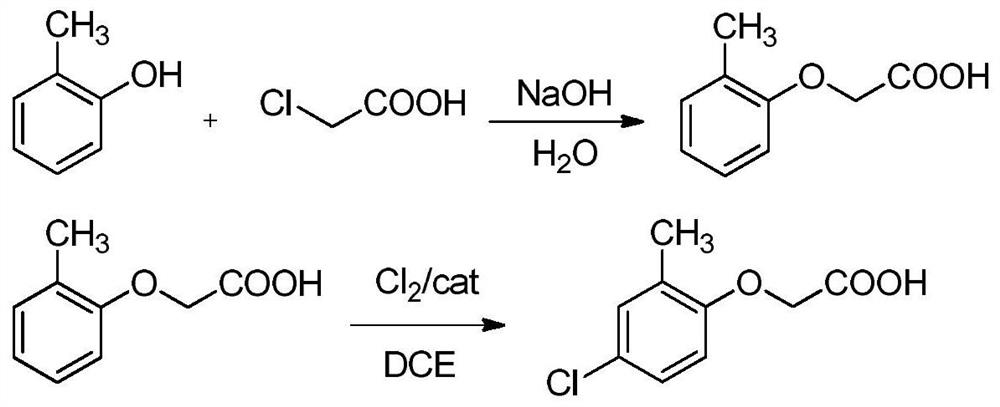
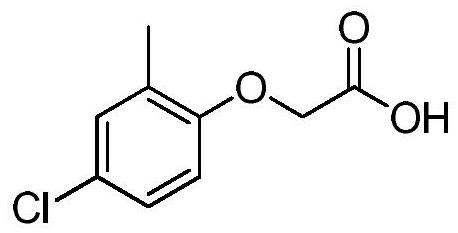
Method for preparing 2-methyl-4-chlorophenoxyacetic acid through catalytic chlorination of 2-methylphenoxyacetic acid
A technology of methylphenoxyacetic acid and o-methylphenoxyacetic acid, applied in the field of preparation of 2-methyl-4-chlorophenoxyacetic acid, can solve problems such as a large amount of chlorinated waste water, achieve good safety and reduce equipment corrosion And the pressure of environmental protection treatment, the effect of avoiding the generation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add 50 g of raw materials o-cresol and 200 g of water into the reaction flask, add 62 g of liquid caustic soda, stir until clarified, then raise the temperature to 120° C., control the same temperature, and add dropwise a pre-prepared sodium chloroacetate aqueous solution (containing 44 g of chloroacetic acid, 100g of water), add in about 5 hours, keep warm for 2 hours until the reaction is complete; add hydrochloric acid to the bottle until the system pH = 1, then add 700ml of dichloroethane for extraction, keep at 50°C, let stand to separate layers, Collect the lower organic phase as the MPA feed solution for the next step reaction.
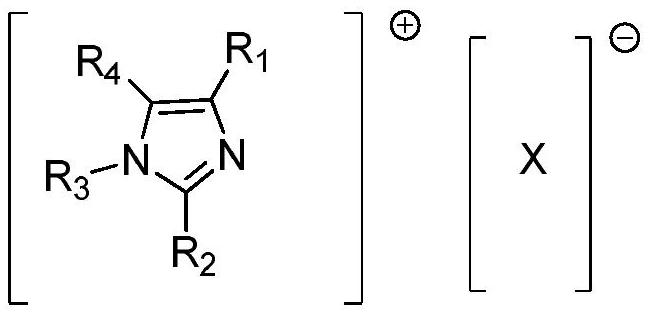
[0046]Add the MPA feed liquid obtained above into the reaction flask, add 0.2 g of the catalyst 1-isopropyl-4-methylimidazole phosphate, start stirring, keep the feed liquid temperature at 70°C, keep warm and start to pass chlorine gas until the raw material is completely converted Stop aeration, consume 35g of chlorine gas, cool to 10°C...
Embodiment 2
[0049] The difference from Example 1 is:
[0050] Add all the MPA solution obtained in Example 1 to the reaction flask, add 1 g of the catalyst 1,3,4-tri-n-pentyl imidazolium tetrafluoroborate, start stirring, keep the temperature of the feed liquid at 30°C, and start to pass chlorine gas while keeping warm until Stop aeration after the conversion of raw materials is complete, consume 47g of chlorine gas, cool down to 5°C, filter, and dry to obtain 84.3g of product MCPA, with a purity of 96% and a yield of 87.3% (calculated as o-cresol).
[0051] Wherein, o-cresol: the weight ratio of chlorine is 1.5.
[0052] EXAMPLES According to the Guidelines for Safety Risk Assessment of Fine Chemical Reactions (Trial Implementation) in the "Guiding Opinions of the State Administration of Work Safety on Strengthening the Safety Risk Assessment of Fine Chemical Reactions", the risk of the reaction process is assessed as Level 1.
Embodiment 3
[0054] The difference from Example 1 is:
[0055] Add all the MPA solution obtained in Example 1 to the reaction flask, add 0.7 g of catalyst 2-ethyl-4-isopropylimidazolium bromide, start stirring, keep the temperature of the material liquid at 58 ° C, keep warm and start to pass chlorine gas until the raw material is converted Completely stop aeration, consume 40g of chlorine gas, cool to 0°C, filter, and dry to obtain product MCPA85.1g with a purity of 96% and a yield of 90.1% (in terms of o-cresol).
[0056] Wherein, o-cresol: the weight ratio of chlorine is 1.2.
[0057] EXAMPLES According to the Guidelines for Safety Risk Assessment of Fine Chemical Reactions (Trial Implementation) in the "Guiding Opinions of the State Administration of Work Safety on Strengthening the Safety Risk Assessment of Fine Chemical Reactions", the risk of the reaction process is assessed as Level 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com