Compound with glucose-reducing and lipid-regulating effects as well as preparation and application thereof
A compound and lipid-regulating technology, applied in the field of medicine and drugs for metabolic diseases of glucose and lipids, can solve problems such as single action, and achieve the effects of improving insulin resistance, excellent in vivo hypoglycemic and lipid-regulating activity, and broad development prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
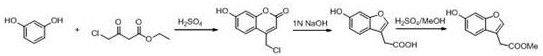
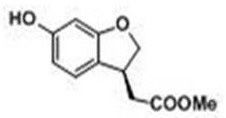
[0021] (6-Hydroxy-1-benzofuran-3-yl)methyl acetate
[0022]
[0023] Ethyl 4-chloroacetoacetate (4.25 ml, 31.43 mmol) was dissolved in 20 ml of concentrated sulfuric acid at 0 °C, and the resulting pale yellow viscous solution was cooled to about -5 °C in an ice bath, and m-benzene was added in batches Diphenol (3.15 g, 28.57mmol), control the internal temperature below 0 °C, after the addition is complete, stir at room temperature for 2 h, the reaction solution is poured into 50ml of ice water, a white solid is precipitated, suction filtered, washed with water (5 ml × 2) The filter cake was dried to obtain 5.6 g of off-white solid, and the crude product yield was 82.3%.
[0024] Take the above crude product (2 g, 9.50 mmol) in a 200 ml single-necked bottle, add 1N NaOH solution (100 ml), the solution immediately turns into a thick yellow, and the above solution is placed in an oil bath and heated to reflux for 2 h. After the reaction is complete, cool to At room temperatu...
Embodiment 2
[0027] (6-Hydroxy-2,3-dihydro-1-benzofuran-3-yl)methyl acetate
[0028]
[0029] Dissolve the raw material ester (2 g, 9.7 mmol) in methanol, add a catalytic amount of palladium carbon 0.2 g, replace it with hydrogen three times, pass in hydrogen and stir at room temperature for 24 h. Cake, and the filtrate was evaporated to remove the solvent under reduced pressure to obtain 1.93 g of off-white powdery solid, with a yield of 95.5%.
[0030] 1 H NMR (300 MHz, CDCl 3 ) δ : 6.97 (d, J = 8.71 Hz, 1H, ArH), 6.31–6.34(m, 2H, ArH), 4.82 (brs, 1H, ArOH), 4.75 (t, J = 9.10 Hz, 1H, -OCH 2 ), 4.26,4.24 (dd, J =5.72, 9.10 Hz, 1H, -OCH 2 ), 3.74–3.84 (m, 1H, ArCH), 3.72 (s, 3H,-OCH 3 ), 2.74, 2.69 (dd, J = 5.72, 16.41 Hz, 1H, -COCH 2 ), 2.55, 2.50 (dd, J =9.11, 16.41 Hz, 1H, -COCH 2 ).
Embodiment 3
[0032] (E)-2-(6-(((3,7-Dimethyloctyl-2,6-dien-1-yl)oxy)-2,3-dihydrobenzofuran-3-yl ) acetic acid (I)
[0033]
[0034] Geraniol (1 g, 6.5 mmol) was added in batches to a pre-cooled 5 ml thionyl chloride solution in dichloromethane under an ice bath, stirred evenly and heated to reflux for 1 h, and the reaction solution was evaporated under reduced pressure to remove excess Thionyl chloride, the resulting brown oil was dissolved in 20 ml THF, added raw material ester (1 equivalent), anhydrous potassium carbonate (3 equivalents), catalytic amount KI, heated to 60 ° C for 8 h, filtered, and reduced pressure The solvent was evaporated, the residue was dissolved in 30 ml of water, extracted with ethyl acetate (20 ml × 3), the organic phases were combined, washed with saturated brine (15 ml × 2), dried over anhydrous sodium sulfate, filtered, and the filtrate was decompressed The solvent was evaporated, and the residue was purified by column chromatography (petroleum ether / ethyl a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com