Nickel metal complex as well as preparation method and application thereof
A technology of metal complexes and reactions, which is applied in the direction of nickel organic compounds, etc., can solve the problems of poor solubility of alkali metal fluoride salts, difficulty in industrial scale-up, and inability to obtain the degree of polymerization, etc., so as to reduce the amount of solvent used, the conditions are not harsh, and the polymerization The effect of cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
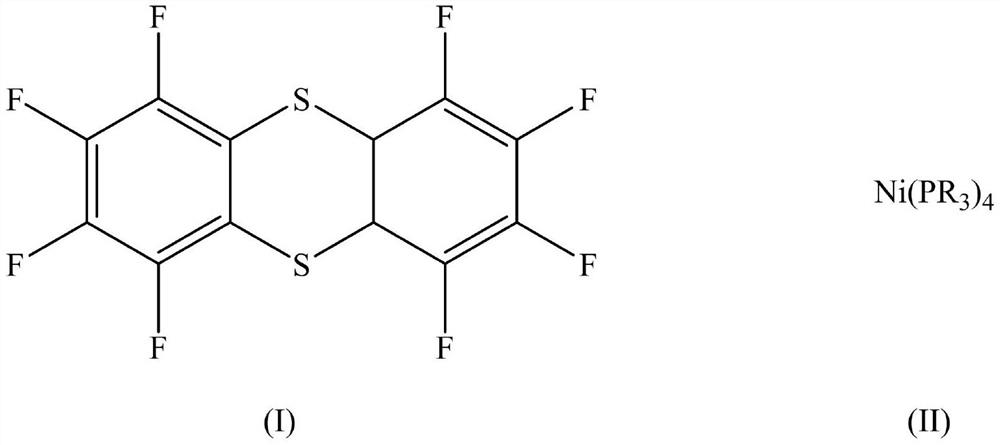
[0034] Raw material A has a structure shown in formula (I);
[0035] Raw material B has a structure shown in formula (II-1);
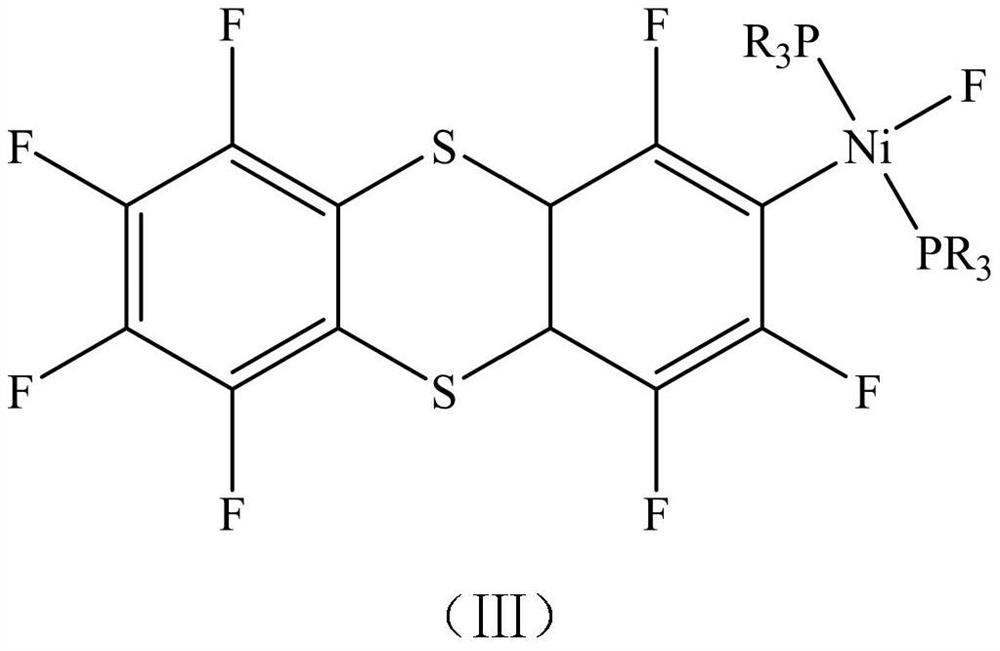
[0036] Catalyst has the structure shown in formula (III-1);
[0037] Step 1: under nitrogen protection system, take by weighing raw material Ni (cod) 2 (21.3mmol), P( i Pr) 3 (37.9mmol) into the reaction system, add 65mL THF solvent, react at 40°C under nitrogen protection for 6h, then cool to room temperature, there is a precipitate, after the reaction is completed, the solid is obtained by suction filtration, washed with n-pentane, and finally placed in a vacuum After drying in a drying oven, raw material B (7.00 g, yield 47%) was obtained as a yellow powder.
[0038] Step 2: Weigh raw material A (13.3mmol), add raw material B (8.0mmol), then add THF 30mL to the system, under nitrogen protection, react at 25°C for 12h, drain the solvent, wash with n-pentane, and remove the remaining solid in Dry in a vacuum oven to obtain a yellow powdery solid ...
Embodiment 2
[0041] Raw material A has a structure shown in formula (I);
[0042] Raw material B has a structure shown in formula (II-1);
[0043] Catalyst has the structure shown in formula (III-1);
[0044] Step 1: under nitrogen protection system, take by weighing raw material Ni (cod) 2 (36.4mmol), P( i Pr) 3 (87.5mmol), then add THF 150mL to the system, react at 40°C for 6h under the protection of nitrogen, then cool to room temperature, there is a precipitate, after the reaction is completed, the solid is obtained by suction filtration, washed with n-pentane, and finally put into a vacuum oven Drying in medium temperature afforded raw material B (13.1 g, yield 63%) as a yellow powder.
[0045] Step 2: Weigh raw material A (22.3mmol), add raw material B (29.0mmol), then add THF 65mL to the system, under nitrogen protection, react at 25°C for 12h, drain the solvent, wash with n-pentane, and remove the remaining solid in Dry in a vacuum oven to obtain a yellow powdery solid (5.11 g...
Embodiment 3
[0048] Raw material A has a structure shown in formula (I);
[0049] Raw material B has a structure shown in formula (II-1);
[0050] Catalyst has the structure shown in formula (III-1);
[0051] Step 1: under nitrogen protection system, take by weighing raw material Ni (cod) 2 (29.5mmol), P( i Pr) 3 (104.5mmol), then added THF 150mL to the system, reacted at 60°C under nitrogen protection for 4h, then cooled to room temperature, and a precipitate was precipitated. After the reaction, the reaction solution was suction filtered, and the solid was washed with n-pentane, and then Put it into a vacuum drying oven and dry to obtain raw material B (10.79 g, yield 64%) as a yellow powder.
[0052] Step 2: Weigh raw material A (21.6mmol), add raw material B (75.6mmol), then add THF120mL to the system, under nitrogen protection, react at 35°C for 9h, drain the solvent, wash with n-pentane, and finally obtain The solid was dried in a vacuum drying oven to finally obtain a yellow po...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com