Immune quantitative detection device, detection method and application of cardiovascular disease markers
A technology of immune quantification and detection method, which is applied in measuring devices, disease diagnosis, biological testing, etc., and can solve the problems that immune detection technology cannot be realized at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
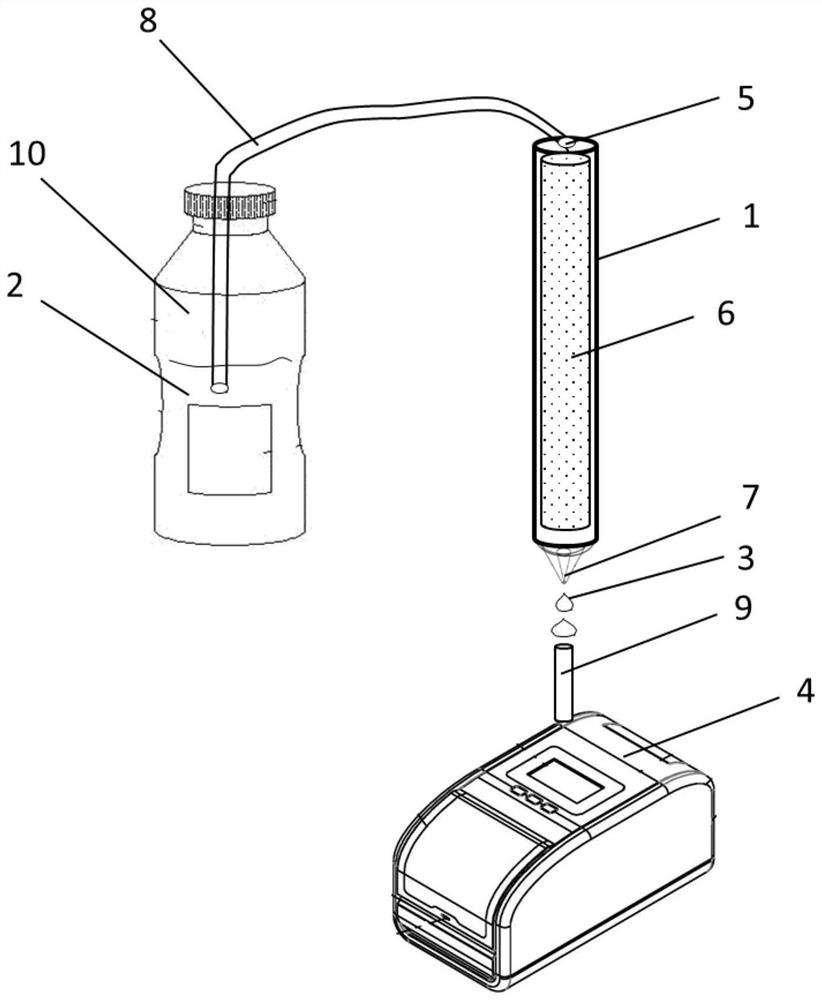
[0071] Embodiment 1: the preparation of filter of the present invention
[0072] Experimental materials: commercially available microfilters, NHS activated agarose gel particles (ACRO BIOSYSTEMS), anti-human fibrinogen polyclonal antibody (Genagates Inc.), sodium bicarbonate, hydrochloric acid, ethanolamine.
[0073] Experimental method: Take a commercially available microfilter and take out the filter element; take 5 mg of anti-human fibrinogen polyclonal antibody plus 0.2M sodium bicarbonate solution, pH 8.3, and dissolve in 2ml; take 1ml of NHS-activated agarose gel particles, and use 20ml of 1mM hydrochloric acid, wash by suction filtration three times; mix the anti-human fibrinogen polyclonal antibody solution with the treated NHS-activated agarose gel particles, and shake for 4 hours at 4°C; the NHS-activated agarose gel after washing reaction gel particles, then add 10mM ethanolamine, 0.2M sodium carbonate solution, pH8.0, shake at room temperature for 4 hours to block ...
Embodiment 2
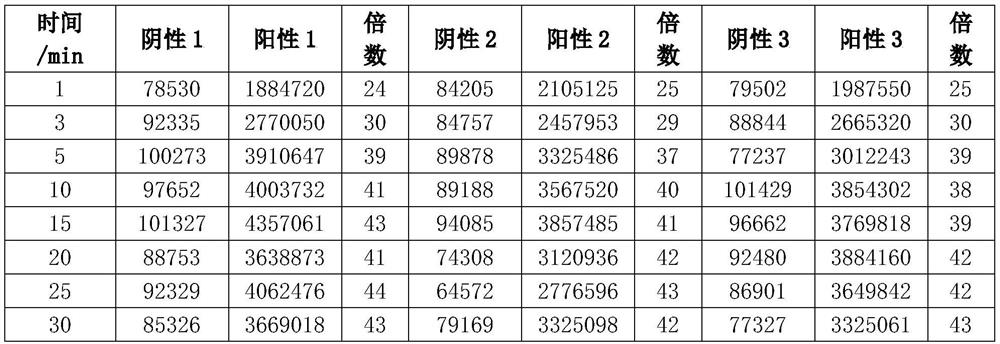
[0074] Embodiment 2: Comparison of detection performance between the present invention and existing chemiluminescence detection technology
[0075] Experimental materials: anti-human fibrinogen polyclonal antibody filter, horseradish peroxidase-labeled anti-human fibrinogen monoclonal antibody, magnetic particles, luminol, p-iodophenol, carbamide peroxide, chemiluminescence detector, human Fibrinogen solution.
[0076] Experimental method: Take human fibrinogen solution of known concentration, dilute it with PBS solution to prepare 1ug / ml human fibrinogen solution. The experimental method will use the present invention and the current chemiluminescence detection technology to observe the influence of different binding reaction times on the amount of luminescence. Observe the binding reaction time points 1, 2, 4, 10, 20, 30, 45, 60 minutes.
[0077] For the current chemiluminescence detection technology group, add 100ul of magnetic particles labeled with anti-human fibrinogen...
Embodiment 3
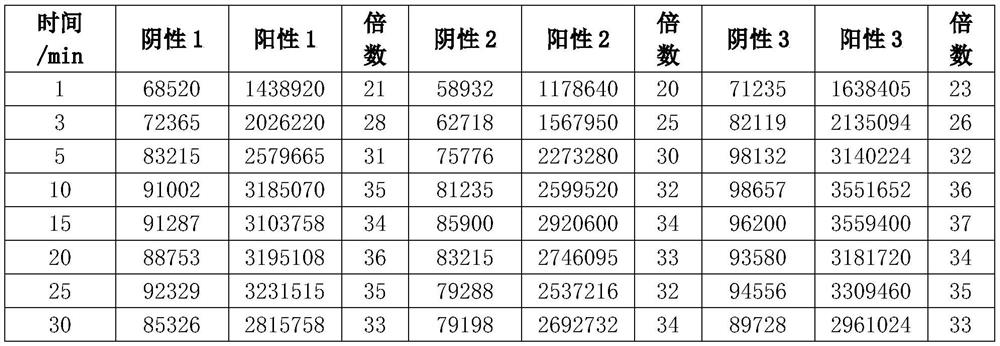
[0080] Embodiment 3: Comparison of the present invention with the detection results of the current chemiluminescence detection technology
[0081] Experimental materials: anti-human fibrinogen polyclonal antibody filter, horseradish peroxidase-labeled anti-human fibrinogen monoclonal antibody, magnetic particles, luminol, p-iodophenol, carbamide peroxide, chemiluminescence detector, human Fibrinogen solution, healthy human plasma.
[0082] Experimental method: take a human fibrinogen standard solution of known concentration, dilute it with PBS solution to prepare 10, 30, 70, 100, 300, 700ng / ml human fibrinogen solution; take another healthy person's plasma, and use PBS for 10000 times Dilution; experimental method adopts the present invention and existing chemiluminescence detection technology to detect human fibrinogen solution and draws standard curve, then measures healthy human fibrinogen, and calculates fibrinogen concentration with standard curve; Get 42 test tubes, be d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com