Method for degrading iodinated drugs by activating sulfite system
A technology for activating sulfite and sulfite, applied in chemical instruments and methods, water pollutants, oxidized water/sewage treatment, etc., can solve the problems of increasing equipment investment and operating costs, high cost of oxidant persulfate, etc., to achieve High electron transfer efficiency, high recycling rate, and cost-saving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] As a method of activating the sulfite system to degrade iodine drugs, the activator selects transition metal sulfide, which can be copper sulfide, cobalt sulfide, manganese sulfide and iron sulfide, etc. Potassium sulfite / sodium / calcium / magnesium is sulfate As the precursor of free radicals and micro-pollutants, iohexol, an iodized X-ray contrast agent, is selected.
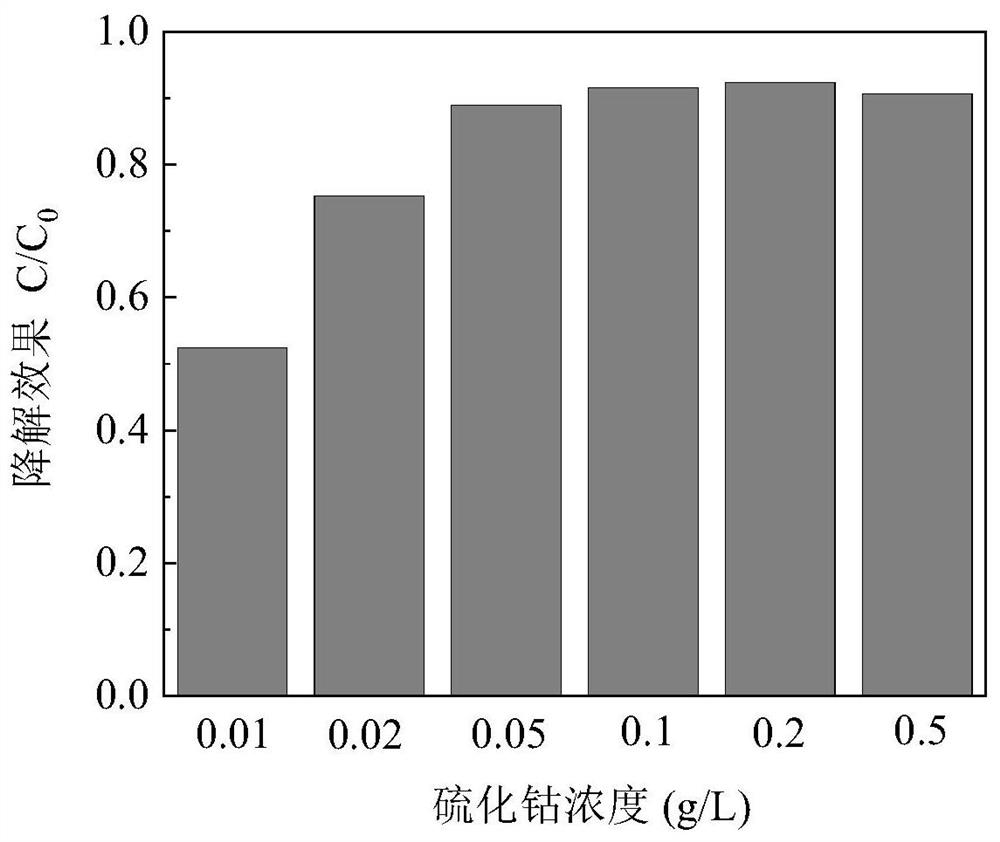
[0027] First, add 0.001g-0.05g of cobalt sulfide to the water to be treated with iohexol as the iodo drug; then, add sulfite to start the reaction and start timing; , 10, 20 and 30 minutes, samples were taken through a 0.22 μm filter membrane to quench the active free radicals in the reaction solution, and the concentration of iohexol was analyzed by ultra-high performance liquid chromatography.
[0028] The effect of different doses of cobalt sulfide on the degradation efficiency of iohexol is as follows: figure 1 As shown, the abscissa represents the dose of cobalt sulfide (g / L), and the ordinate repres...
Embodiment 2
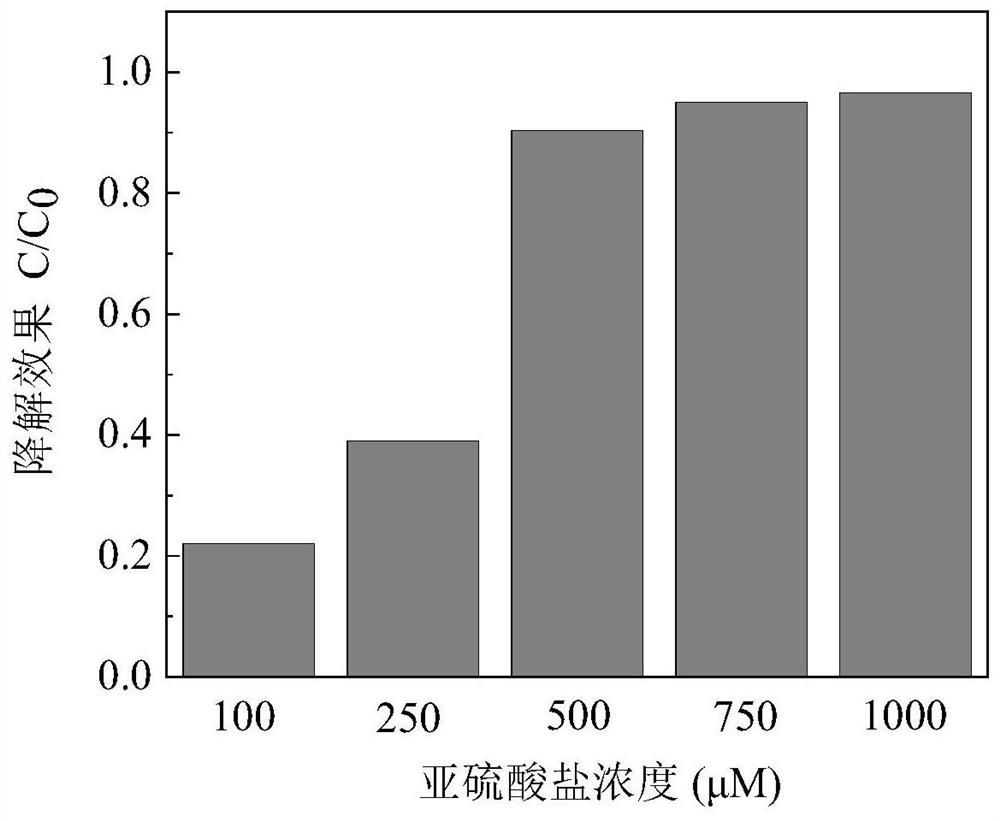
[0030] The difference between this embodiment and specific example 1 is: through the optimization of cobalt sulfide concentration in embodiment 1, the concentration of cobalt sulfide is finally selected to be 0.05g / L; in the present embodiment, the concentration range of sulfite is 100- 1000 μM.
[0031] The effect of different sulfite concentrations on the degradation efficiency of iohexol was as follows: figure 2 As shown, the abscissa indicates the concentration of sulfite (μM), and the ordinate indicates the degradation of iohexol (C / C 0 ). In this example, the concentration of sulfite was optimized, and the degradation of iohexol at concentrations of 100, 250, 500, 750, and 1000 μM were performed respectively, wherein the dose of cobalt sulfide was 0.05 g / L, and iohexol The concentration is 10 μM, and the pH value is 8. The result shows: the raising of sulfite concentration, promptly the sulfite ion concentration that can participate in reaction raises in the system, ...
Embodiment 3
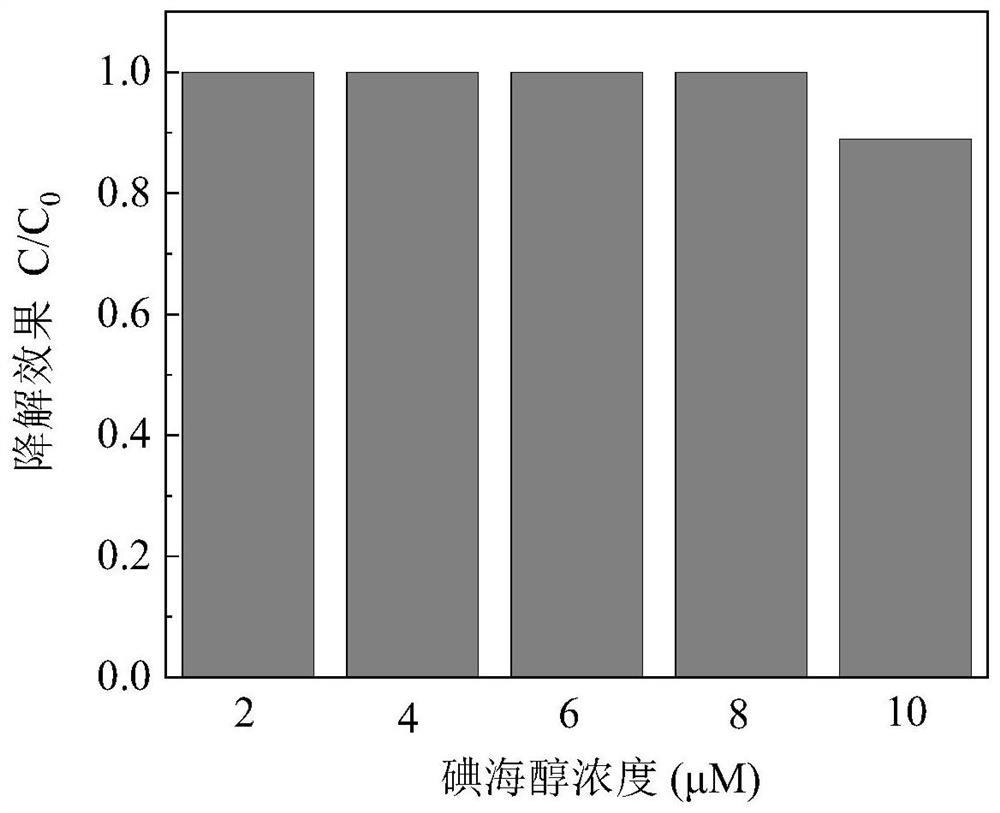
[0033] The difference between this embodiment and the specific example 2 is: through the optimization of the sulfite concentration in Example 2, the concentration of sulfite is finally selected as 500 μM; in this example, the concentration range of iohexol is 2-10 μM .
[0034] The effect of different iohexol concentrations on the degradation efficiency of iohexol is as follows: image 3 As shown, the abscissa represents the concentration of iohexol (μM), and the ordinate represents the degradation of iohexol (C / C 0 ). In this example, different concentrations of iohexol were degraded, and degradations were performed at concentrations of 2, 4, 6, 8, and 10 μM respectively, wherein the dose of cobalt sulfide was 0.05 g / L, and the concentration of sulfite 10 μM, pH 8. The results show that: when the concentration of iohexol is 2-8μM, the degradation effect of the system on iohexol can reach 100%; when the concentration of pollutants is 10μM, the degradation efficiency is ~89%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com