Iron oxide/boron nitride nano-catalyst as well as preparation method and application thereof
A catalyst, boron nitride technology, applied in the field of catalysis, can solve the problems of poor size adjustable variability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The present invention additionally provides the preparation method of above-mentioned catalyst, described method comprises:
[0039] (1) preparing inorganic iron salt solution and boron nitride dispersion respectively; (2) adding the inorganic iron salt solution into the boron nitride dispersion to obtain a suspension; subjecting the suspension to a solvothermal reaction at high temperature to obtain a nanocomposite material comprising boron nitride and iron oxide, and washing, centrifuging, and drying the nanocomposite material; and optionally (4) performing step (3) The resulting dried nanocomposite is calcined to obtain the catalyst.
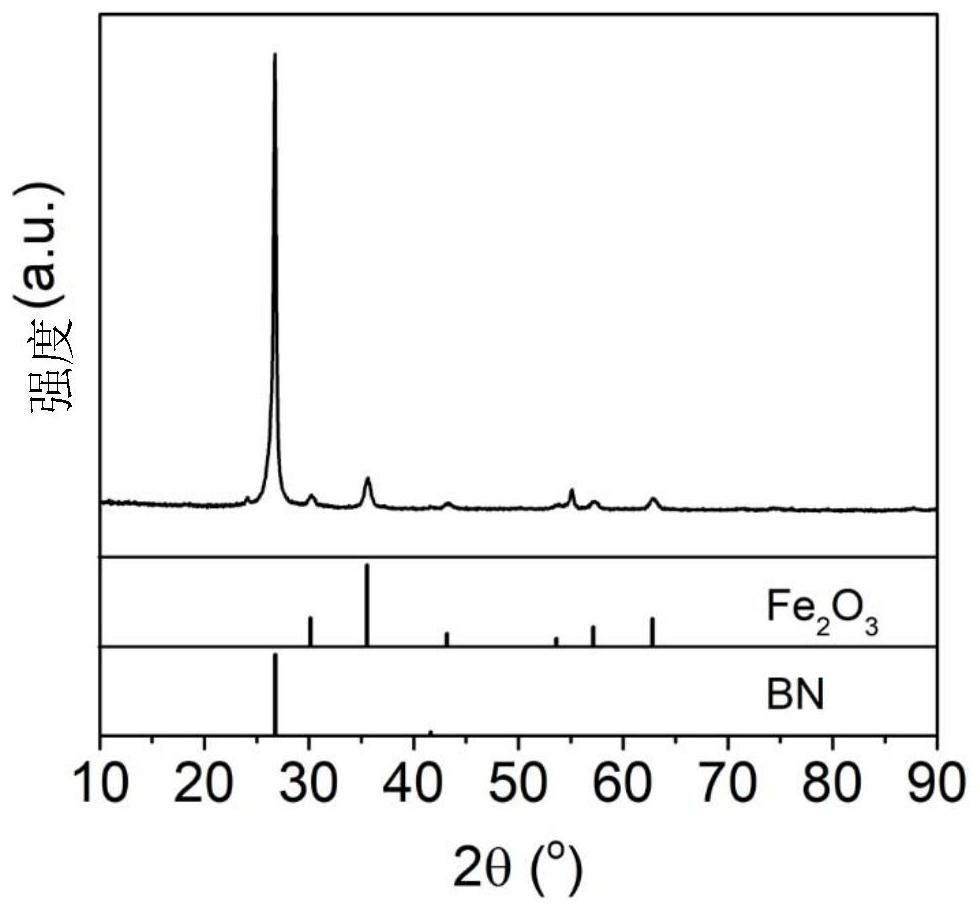
[0040] The above preparation method of the present invention is based on the following principle: the formation of iron oxide nanoparticles on the surface of hexagonal boron nitride follows a nucleation and growth mechanism. Oxygen-containing functional groups (hydroxyl groups, etc.) on the surface of boron nitride make the surface of...
Embodiment 1
[0127] Embodiment 1: preparation of catalyst of the present invention and Fischer-Tropsch synthesis performance test
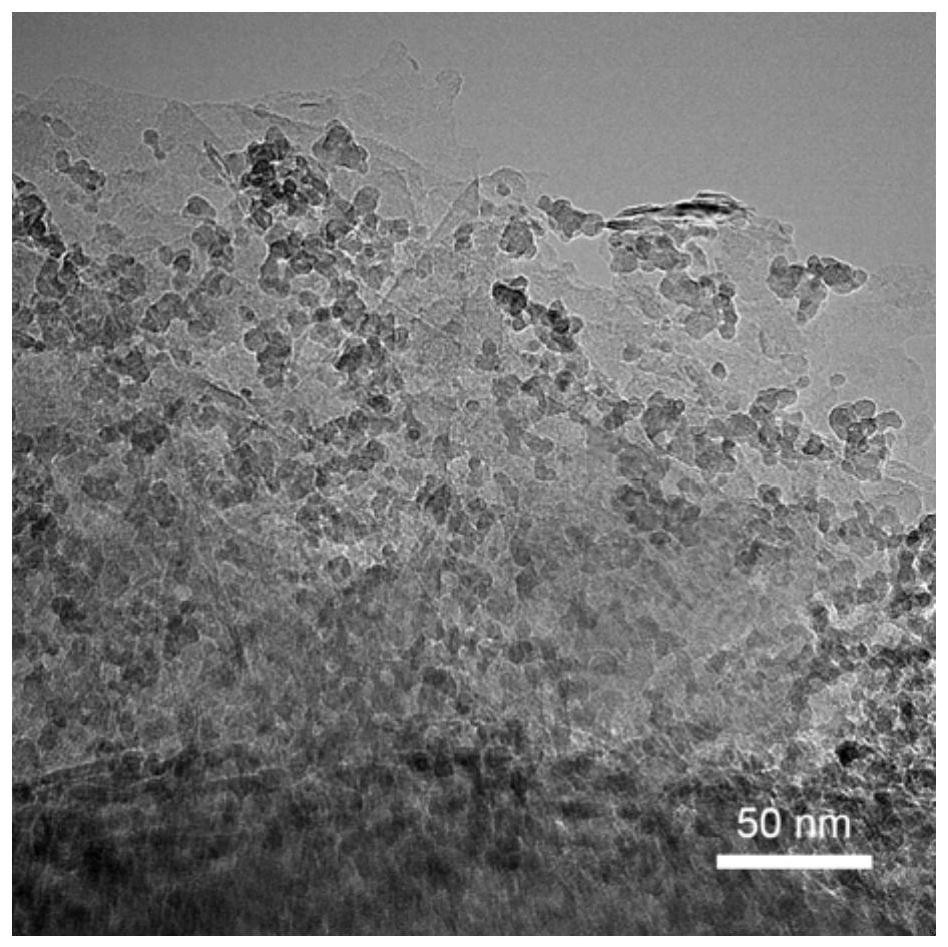
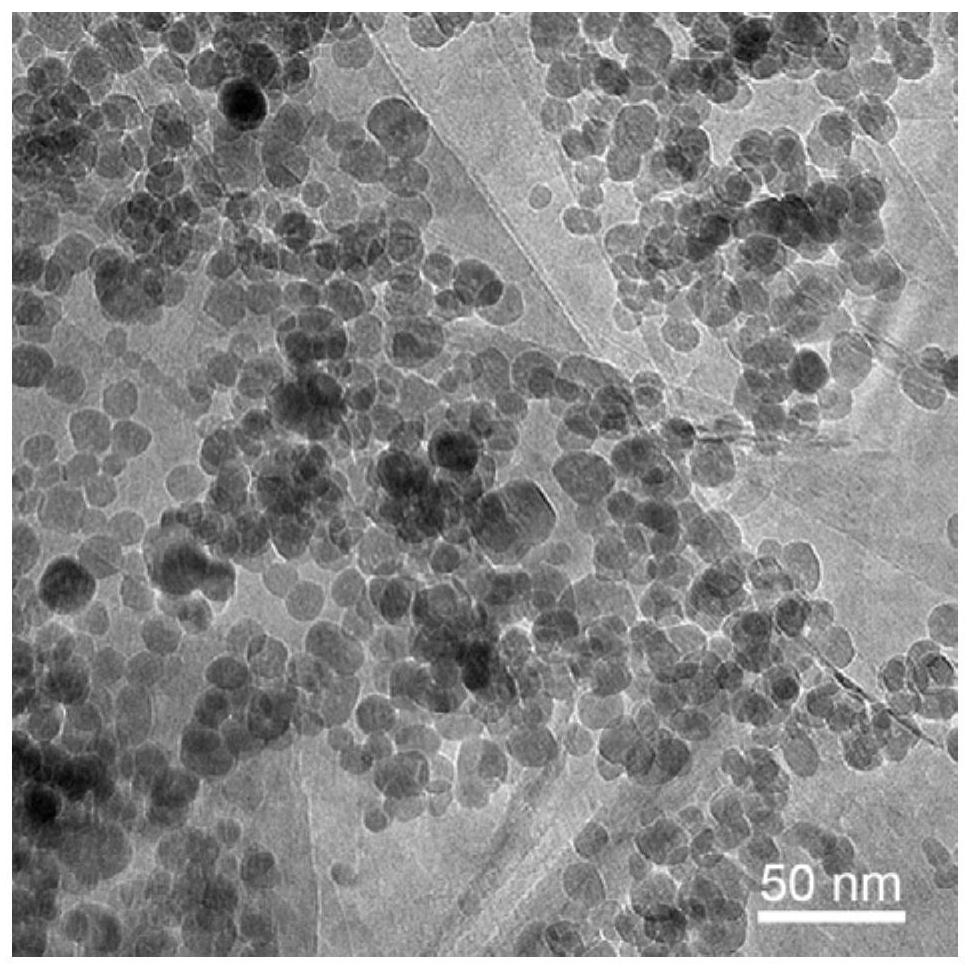
[0128] (1) Dissolve ferric nitrate in ethylene glycol and stir until it is completely dissolved to obtain 0.25mol / L and 0.5mol / L ferric nitrate solutions. The ethylene glycol dispersion liquid of the hexagonal phase boron nitride of preparation 5.0g / L; Wherein, the specific surface area of described hexagonal phase boron nitride is 105m 2 / g, the particle size is 400nm.
[0129] (2) 53.6 mL of ferric nitrate solution in step (1) is added dropwise in the ethylene glycol dispersion of 300 mL of boron nitride, while mechanically stirring to obtain a stable and uniform suspension; The mass ratio of element) to hexagonal boron nitride is 50:100, 100:100.
[0130] (3) Transfer the suspension in step (2) to the reaction kettle, react at 180°C for 12h, after the reaction is finished, cool to room temperature naturally, wash with deionized water, centrifuge 3-5 tim...
Embodiment 2
[0134] Embodiment 2: preparation of catalyst of the present invention and Fischer-Tropsch synthesis performance test
[0135] (1) Dissolve ferrous sulfate in dimethylformamide, stir until it dissolves completely, obtain 0.05mol / L ferrous sulfate solution; and prepare the toluene dispersion of 3.0g / L hexagonal phase boron nitride; wherein, The specific surface area of the hexagonal phase boron nitride is 140m 2 / g, the particle size is 100nm.
[0136] (2) Add 41mL of the iron salt solution in step (1) dropwise to the toluene dispersion of 383mL of boron nitride, and magnetically stir at the same time to obtain a stable and uniform dispersion; wherein the active metal iron (calculated as iron element ) to the mass ratio of hexagonal boron nitride is 10:100.
[0137] (3) Transfer the suspension in step (2) to the reactor, and react at 140°C for 24h. After the reaction, cool down to room temperature naturally, wash with deionized water, and centrifuge 5 to 8 times. In a tube f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com