Preparation method of fluorine-containing compound for hydrophobic surface treatment agent
A hydrophobic surface and treatment agent technology, which is applied in the field of preparation of fluorine-containing compounds for hydrophobic surface treatment agents, can solve problems such as complex preparation methods, and achieve the effect of being suitable for large-scale production and avoiding oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
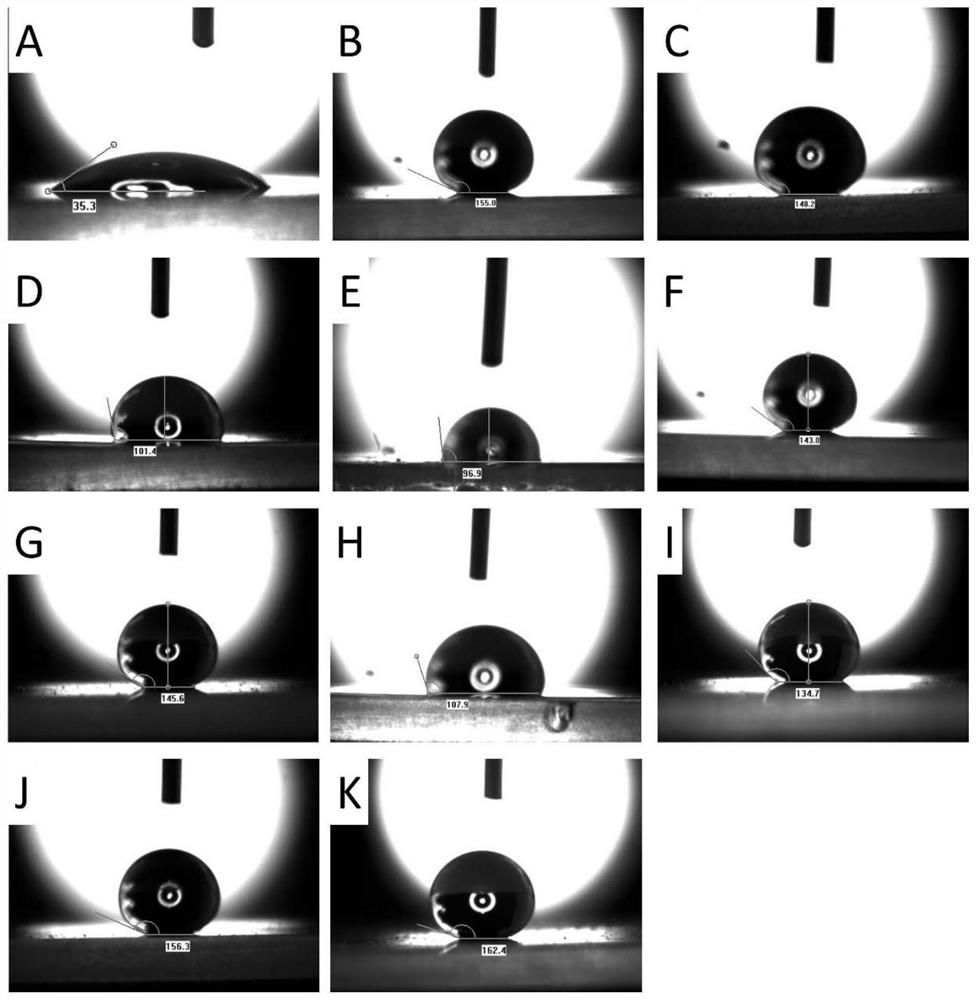
Image
Examples
preparation example Construction
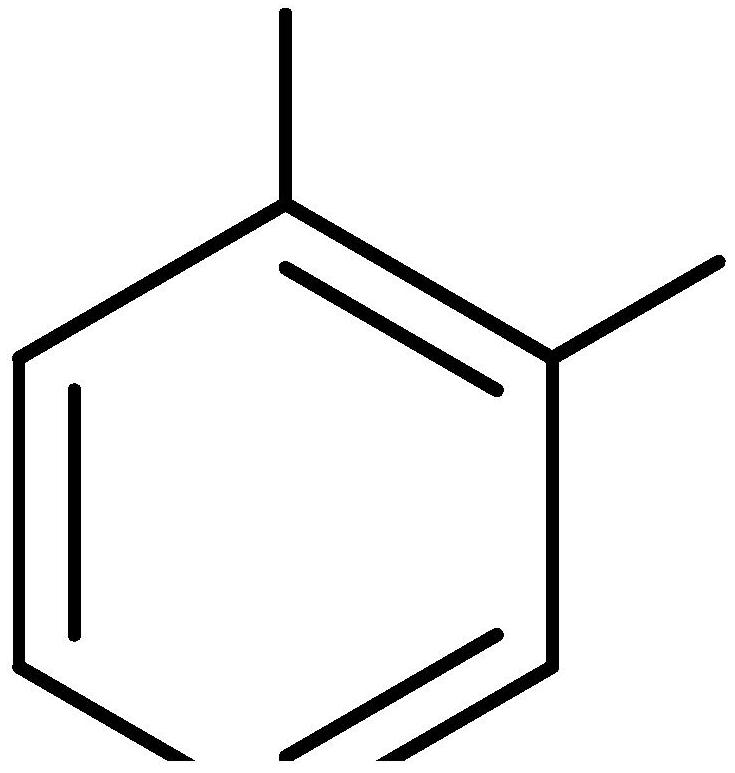
[0031] The invention provides a preparation method of a fluorine-containing compound for a hydrophobic surface treatment agent. Dopa is used as a starting material, and its functional group is protected by a benzyl chloride reagent or a benzyl bromide reagent, and then the carboxyl protection is removed by alcoholysis. Then carry out esterification or amidation reaction with long-chain perfluoroalkanols or alkylamines, and finally deprotect to obtain long-chain perfluoroalkanes based on the dopa structure. The typical reaction route is as follows:
[0032]
[0033] Specific steps are as follows:
[0034] 1) Group protection
[0035] Use benzyl chloride reagents or benzyl bromide reagents to react with dopa in polar organic solvents, and add an acid-binding agent to protect the phenolic hydroxyl and amino groups of dopa molecules, specifically:
[0036] Dopa (3,4-dihydroxyphenylalanine) is completely dissolved in a polar organic solvent, and the polar organic solvent prefer...
Embodiment 1
[0067] 1) Dissolve 1.97g levodopa in 30ml N,N-dimethylformamide, then add 8.5g 4-methoxybenzyl chloride and 9g potassium carbonate (K 2 CO 3 ), under the protection of nitrogen, after stirring and reacting for 12h under the temperature condition of 60°C, 100ml of dichloromethane and 100ml of water were added to the reaction solution for extraction, the organic phase was separated and washed 3 times, and the solvent was evaporated to dryness Afterwards, a light yellow viscous product is obtained;
[0068] 2) Dissolve 5g of the product from the previous step in 50ml of methanol, add 1g of potassium hydroxide and 2ml of water, then reflux for 6 hours, add dilute hydrochloric acid dropwise after the reaction to adjust the pH to about 4.0, evaporate the solvent and add 100ml of water , extracted 3 times with dichloromethane, and evaporated to dryness to obtain a light yellow solid;
[0069] 3) Dissolve 1.36g of the light yellow solid in the previous step in 100ml of dichlorometha...
Embodiment 2
[0077] The difference with embodiment 1 is:
[0078] In step 1), the solvent N,N-dimethylformamide is replaced by dimethyl sulfoxide, and the addition is still 30ml; 4-methoxybenzyl chloride is replaced by 4-methoxybenzyl bromide, and the addition is 11 g; replace potassium carbonate with cesium carbonate (Cs 2 CO 3 ), the addition amount is 18g; the reaction environment is adjusted to react at 0°C for 1h, and then react at 50°C for 2h;
[0079] In step 2), methanol is replaced by ethanol, and the addition is still 50ml; potassium hydroxide is replaced by sodium hydroxide, and the addition is still 1g;
[0080] In step 3), the solvent dichloromethane is replaced by chloroform, and 2-perfluorooctyl ethanol is replaced by 1H, 1H-perfluoro-1-nonanol, and the addition amount is 0.85g; 1-(3-di The catalytic system of methylaminopropyl)-3-ethylcarbodiimide (EDC1) / 1-hydroxybenzotriazole (HOBt) / 4-dimethylaminopyridine (DMAP) was replaced by dicyclohexylcarbodiimide Imine (DCC) / 4-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com