Alkaline aqueous mixed flow battery based on electroactive phenazine derivative negative electrode
A mixed liquid and flow battery technology, applied in the direction of regenerative fuel cells, fuel cells, battery electrodes, etc., can solve the problems of low specific capacity of solid-state electroactive organic molecular electrodes, and achieve high rate performance, high specific capacity and effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis and Electrochemical Properties of Anode Material 5,6,11,12,17,18-hexaazatrinaphthalene
[0037] 5,6,11,12,17,18-hexaazatrinaphthalene was synthesized by the Schiff base condensation reaction of cyclohexanone and 1,2-phenylenediamine (Acta Cryst.2001, E57, o242-o244). Cyclohexanone octahydrate (0.8 mmol) and 1,2-phenylenediamine (2.6 mmol) were mixed and co-dissolved in 50 mL of absolute ethanol. After refluxing for 12 hours under a nitrogen atmosphere, cool to room temperature. The initial product was collected by vacuum filtration as an orange solid. Recrystallized with chloroform, washed with water, ethyl acetate and acetone in sequence, and dried in vacuum at 60°C for 24 hours to obtain the product with a yield of about 78%.
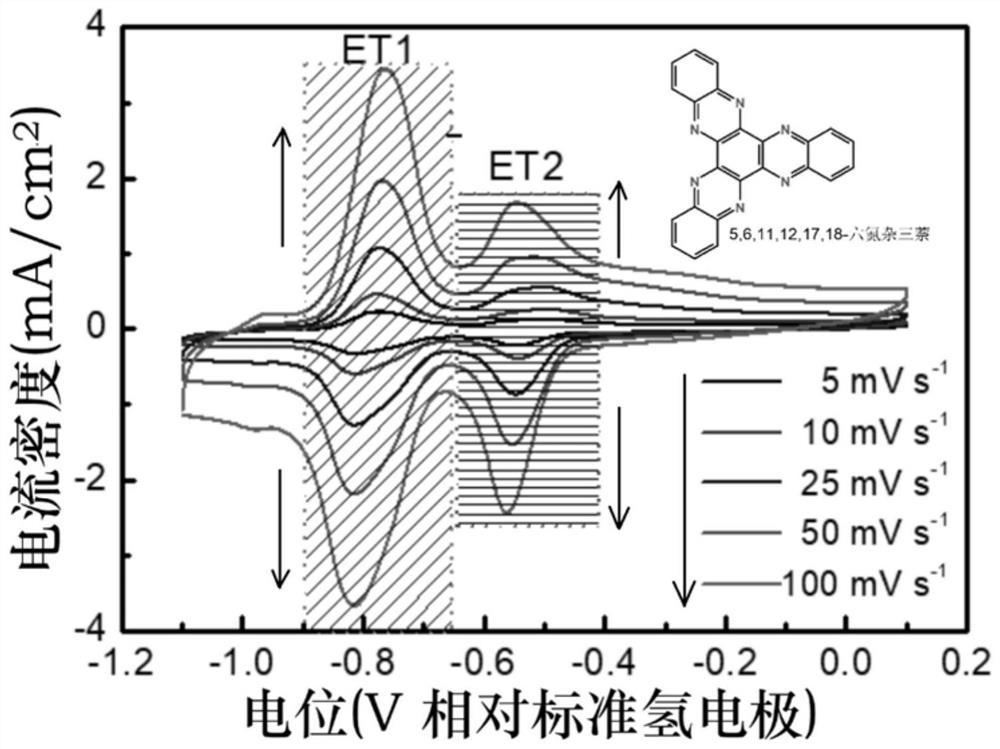
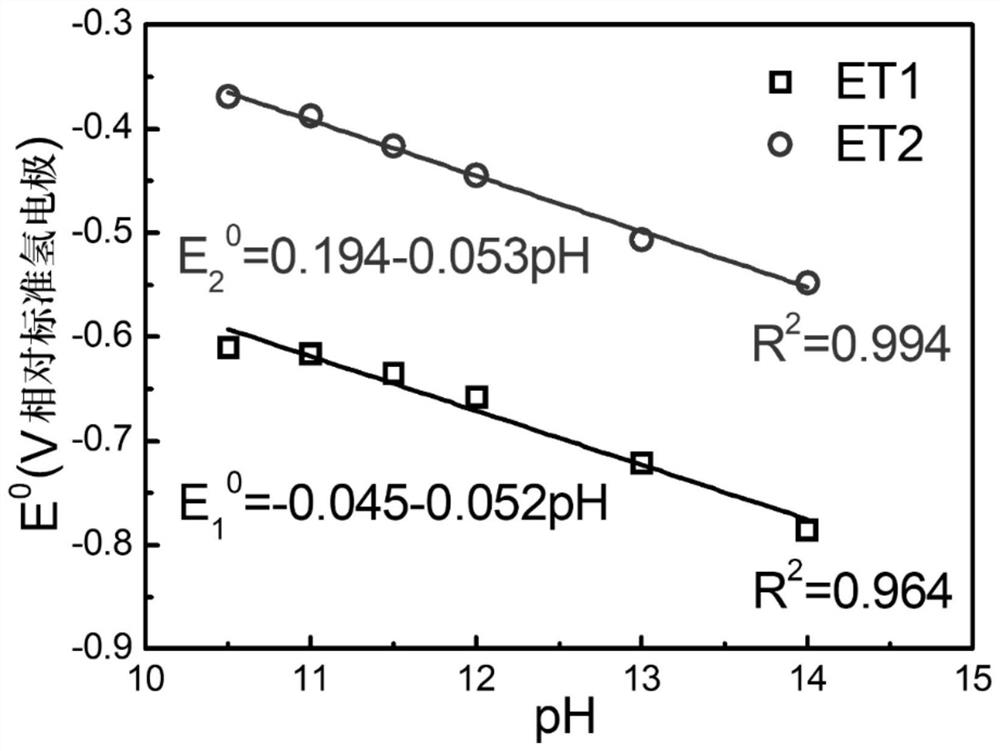
[0038] The electrochemical properties of 5,6,11,12,17,18-hexaazatrinaphthalene were characterized by cyclic voltammetry. Mix 2 mg of 5,6,11,12,17,18-hexaazatrinaphthalene and 2 mg of Ketjen Black (Ketjen Black) carbon powder, add 0...
Embodiment 2
[0041] Example 2 Synthesis and electrochemical properties of negative electrode material 1,5,9-tribromo-5,6,11,12,17,18-hexaazatrinaphthalene
[0042]The synthetic method of 1,5,9-tribromo-5,6,11,12,17,18-hexaazatrinaphthalene is the same as that of 15,6,11,12,17,18-hexaazatrinaphthalene in Example 1 Trinaphthalene. Cyclohexaone octahydrate (0.8 mmol) and 1,2-diamino-3-bromobenzene (2.6 mmol) were mixed and co-dissolved in 50 ml of absolute ethanol. After reflux reaction under nitrogen atmosphere for 12 hours, it was cooled to room temperature. The initial product was collected by vacuum filtration as an orange solid. Recrystallized with chloroform, washed with water, ethyl acetate and acetone in sequence, and dried in vacuum at 60°C for 24 hours to obtain the product with a yield of about 65%.
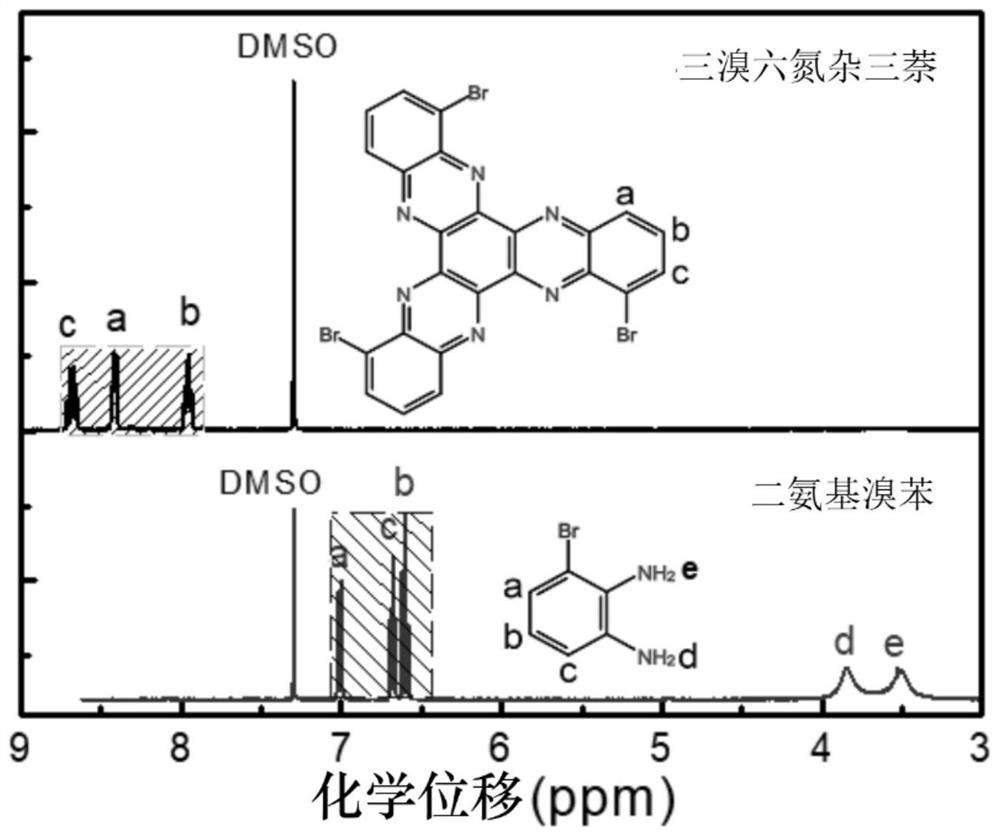
[0043] image 3 It is the H NMR spectrum of 1,5,9-tribromo-5,6,11,12,17,18-hexaazatrinaphthalene. The electrochemical properties of 1,5,9-tribromo-5,6,11,12,17,18-hexaazatrinapht...
Embodiment 3
[0045] Example 3 negative electrode material 1,2:3,4-dibenzophenazine, dibenzo[a,c]phenazine-11-amine and tribenzo[a,c,i]-phenazine-10, Electrochemical properties of 15-diketones
[0046] 1,2:3,4-Dibenzophenazine, dibenzo[a,c]phenazin-11-amine and tribenzo[a,c,i]-phenazine were studied by cyclic voltammetry (CV) Electrochemical properties of oxazine-10,15-dione. The preparation and electrochemical testing steps of the working electrode are the same as in Example 1.
[0047] Figure 5 It is the cyclic voltammogram of 1,2:3,4-dibenzophenazine in 1mol / L KOH. The CV diagram of 1,2:3,4-dibenzophenazine shows a pair of reversible redox peaks with a standard equilibrium potential of -0.78V.
[0048] Figure 6 It is the cyclic voltammogram of dibenzo[a,c]phenazin-11-amine in 1mol / L KOH. The CV plot of dibenzo[a,c]phenazin-11-amine showed a pair of well-shaped redox peaks with a standard equilibrium potential of -0.825V.
[0049] Figure 7 is the cyclic voltammogram of tribenzo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Peak power density | aaaaa | aaaaa |
| Peak power density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com