Preparation method of triphenylene derivative
A technology for triphenylene and derivatives, applied in the field of preparation of triphenylene derivatives, can solve the problems of not being an industrialized production method, low efficiency and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
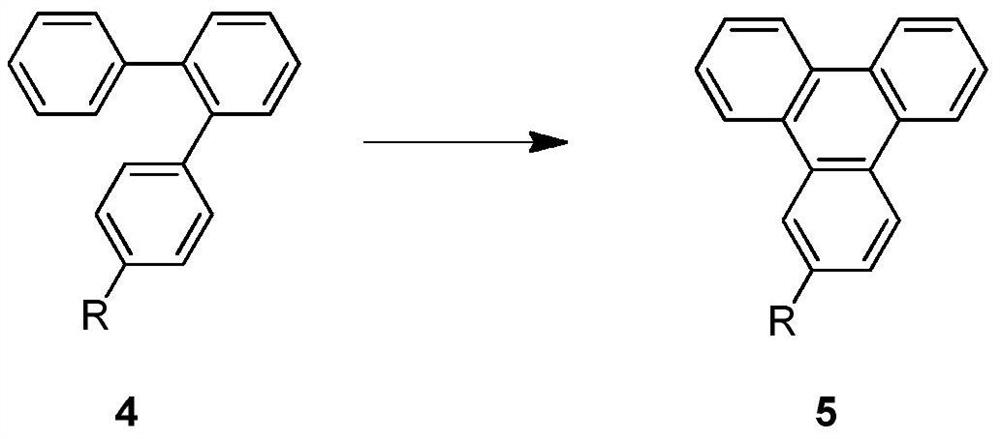
[0059] The present invention provides a preparation method of triphenylene derivatives, comprising: subjecting the compound of formula 4 to ring-closing reaction in the presence of an acid, an initiator and an oxidizing agent to provide the compound of formula 5, and the reaction equation is as follows:
[0060]
[0061] Wherein, R is selected from Cl, Br, OR', and R' is selected from H, C1-C5 alkyl, benzyl. For the compound of formula 4, there is a problem of low reactivity when it is used as a reaction substrate, so the commonly used scheme of oxidative ring closure by oxidizing agent is difficult to realize efficiently on this type of substrate. However, in the present invention, by introducing an initiator to excite the intermediate state of the reaction, the reaction can smoothly cross the reaction energy barrier, thereby successfully completing the ring closure reaction.
[0062] In the present invention, the "alkyl group" generally refers to a saturated aliphatic gro...
Embodiment 1
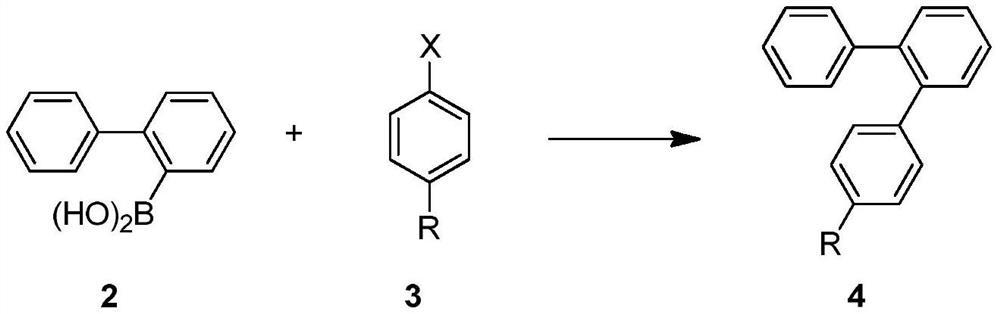
[0101] Preparation of biphenyl-2-boronic acid:
[0102] Add 28.8g (1.2mol) metal magnesium and a grain of iodine to a 2000ml four-necked bottle, dissolve 233g (1.0mol) of 2-bromobiphenyl in 1100ml THF, add it to a constant pressure dropping funnel, and add dropwise 50ml of this mixture In the reaction bottle, heat to initiate the format, after the initiation, control the temperature between 50°C and 55°C and add the remaining 2-bromobiphenyl solution dropwise. Lower the temperature of the system to -40°C, add dropwise a solution of 135.2g (1.3mol) trimethyl borate dissolved in 240ml THF, control the temperature to less than -25°C, keep warm at this temperature for 1 hour after the dropwise addition, and then naturally rise to room temperature, Add hydrochloric acid dropwise to adjust PH = 1, separate layers, distill off THF under reduced pressure, add water for beating, filter with suction, add petroleum ether to the filter cake for beating, and dry to obtain 163g of biphenyl-...
Embodiment 2
[0105] Preparation of biphenyl-2-boronic acid:
[0106]Add 233g (1.0mol) of 2-bromobiphenyl and 1400ml THF into a 5000ml four-necked bottle, add butyllithium solution (1.2mol) dropwise between -50°C and -65°C, keep warm for 1 hour after dropping, and monitor by gas chromatography Raw materials are reacted. Add 244.5g (1.3mol) triisopropyl borate dropwise at -50°C, control the temperature below -5°C, keep warm at this temperature for 1 hour after the dropwise addition, then naturally rise to room temperature, add hydrochloric acid dropwise to adjust PH=1, and separate layers , THF was evaporated under reduced pressure, water was added for beating, suction filtration was added, and toluene was added for beating the filter cake, and 169 g of biphenyl-2-boric acid was obtained by drying, the liquid chromatography content was 99.3%, and the yield was 85.5%.
[0107]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com