Preparation method of lithium difluorophosphate
A technology of lithium difluorophosphate and lithium hexafluorophosphate, which is applied in chemical instruments and methods, phosphorus compounds, secondary batteries, etc., can solve the problems of high preparation cost of lithium difluorophosphate, difficulty in purifying lithium difluorophosphate, and difficult control of the reaction process. , to achieve the effect of being beneficial to actual operation, slowing down, and easy to separate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
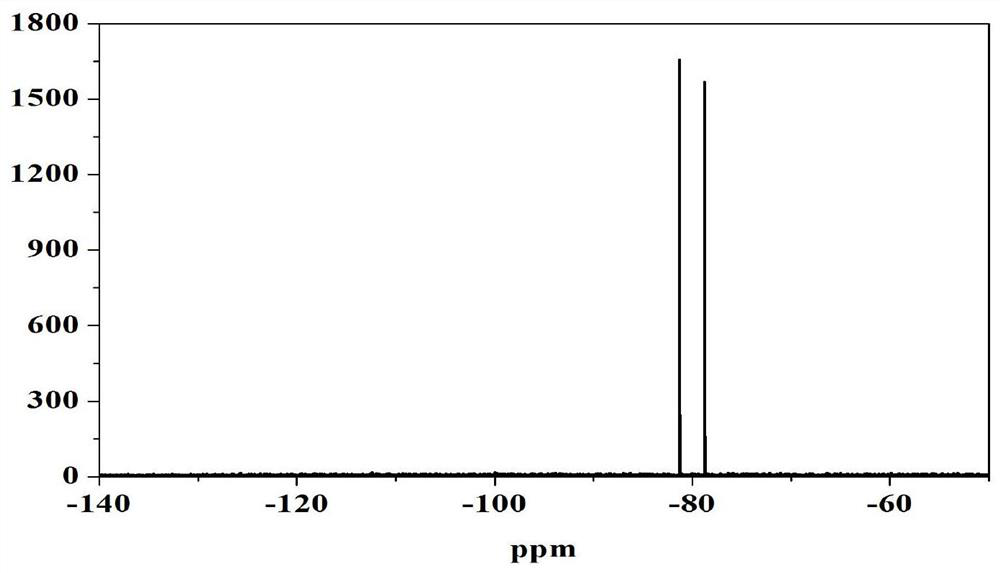
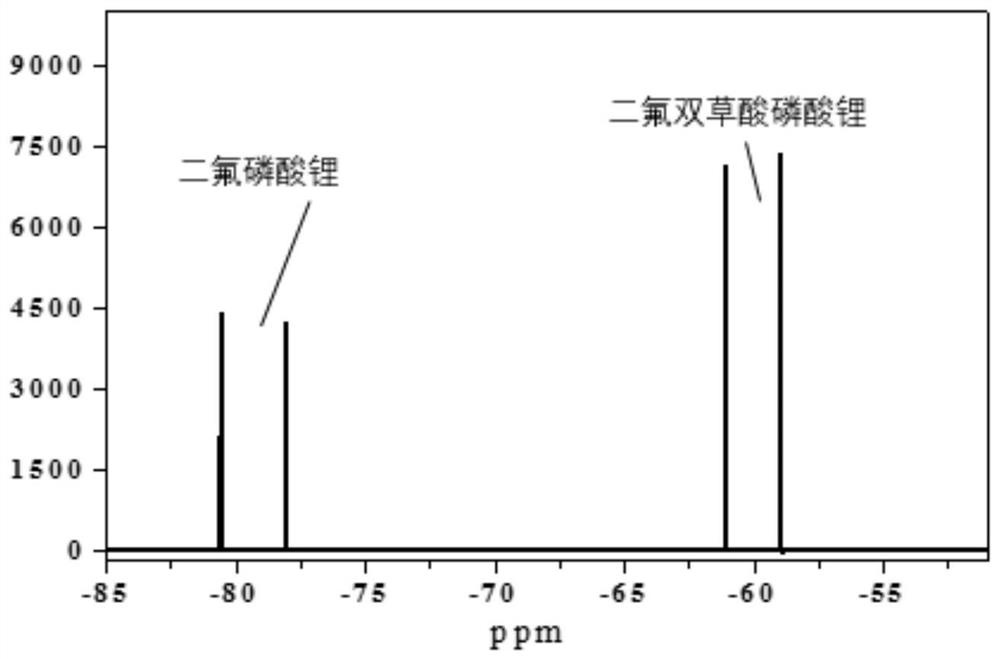
Image
Examples
Embodiment 1
[0037] Prepare a 1L two-neck flask, put it in the glove box, add 300mL dimethyl carbonate, weigh 50g LiPF 6 , which was slowly added into dimethyl carbonate, and the flask was taken out after stirring for 2 h. Place it on a magnetic stirrer, then weigh 40.8g of ammonium oxalate, quickly add it to the flask, and then measure 21mL of SiCl 4 , quickly transferred to the flask, and the reaction occurred immediately. At this time, after replacing the air in the flask with nitrogen, connect the tail gas absorption device, and react at room temperature for 8 hours, almost no bubbles emerge, and the reaction ends. Filter to obtain a filter cake containing lithium difluorophosphate and ammonium chloride, add the filter cake to a flask containing 400 mL of ethyl acetate, and stir at room temperature for 4 h. Then filter to obtain an ethyl acetate solution containing only lithium difluorophosphate. Distill the solution under reduced pressure at 50°C. When concentrated under reduced pre...
Embodiment 2
[0039] Prepare a 1L two-neck flask, put it in the glove box, add 300mL of dichloromethane, weigh 50g of LiPF 6, which was slowly added to dichloromethane, and the flask was taken out after stirring for 2 h. Place it on a magnetic stirrer, then weigh 40.8g of ammonium oxalate, quickly add it to the flask, and then measure 21mL of SiCl 4 , quickly transferred to the flask, and the reaction occurred immediately. At this time, after replacing the air in the flask with nitrogen, connect the tail gas absorption device, and react at room temperature for 8 hours, almost no bubbles emerge, and the reaction ends. Filter to obtain a filter cake containing lithium difluorophosphate and ammonium chloride, add the filter cake to a flask containing 380 mL of ethyl acetate, and stir at room temperature for 5 h. Then filter to obtain an ethyl acetate solution containing only lithium difluorophosphate, distill the solution under reduced pressure at 50°C, and concentrate under reduced pressure...
Embodiment 3
[0041] Prepare a 2L three-neck flask, put it in the glove box, add 800mL toluene, and weigh 100g LiPF 6 , which was slowly added to toluene, and the flask was taken out after stirring for 3 h. Place it on a magnetic stirrer, then weigh 131g of potassium oxalate, quickly add it to the flask, and then measure 46mL of SiCl 4 , quickly transferred to the flask, and the reaction occurred immediately. At this time, after replacing the air in the flask with nitrogen, connect the tail gas absorption device, and react at room temperature for 20 hours, almost no bubbles emerge, and the reaction ends. Filter to obtain a filter cake containing lithium difluorophosphate and potassium chloride, add the filter cake to a flask containing 850 mL of methanol, and stir at room temperature for 3 h. Then filter to obtain a methanol solution containing only lithium difluorophosphate, distill the solution under reduced pressure at 60°C, concentrate under reduced pressure to just saturated state, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com