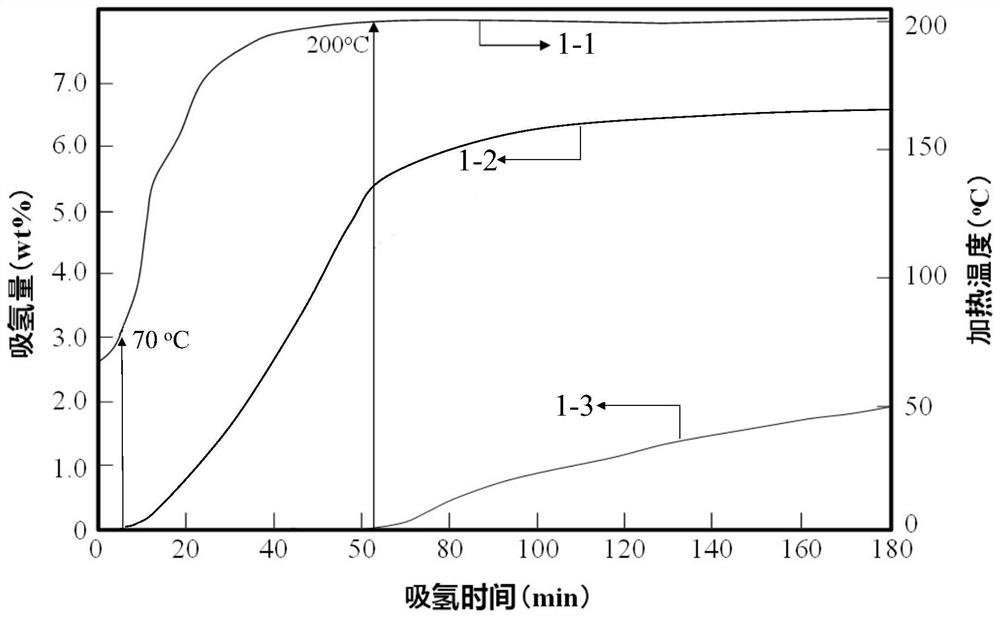
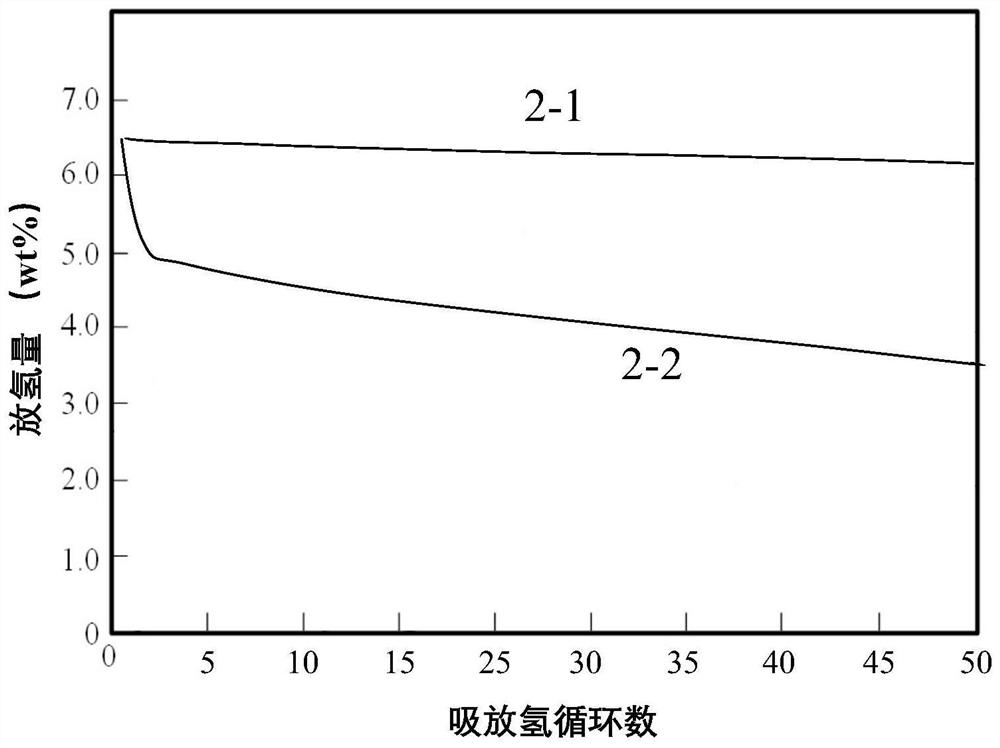
Preparation method of magnesium-based hydrogen storage material wrapped by rare earth oxide and nano boron nickel
A technology of rare earth oxides and rare earth hydroxides, applied in chemical instruments and methods, control of reactant parameters, hydrogen, etc., can solve problems such as high hydrogen absorption and desorption temperature, decreased hydrogen storage capacity, and increased alloy density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: mixed nitrate solution preparation
[0026] Dissolve 1 mole of nickel nitrate and 0.5 mole of lanthanum nitrate in 1.8 liters of deionized water at a molar ratio of 1:0.5:100 to form a nickel and lanthanum nitrate solution.
Embodiment 2
[0027] Embodiment 2: the formation of nanometer amorphous boron nickel
[0028] Dissolve 1 mole of nickel nitrate and 1 mole of cerium nitrate in 1.8 liters of deionized water at a molar ratio of 1:1:100 to form a nickel and cerium nitrate solution. 0.25 mole of sodium borohydride (9.45 g) was dissolved in 463.05 g of 10 wt % NaOH solution to obtain a lye solution containing 2 wt % sodium borohydride. Sodium borohydride lye is added dropwise to the nitrate solution of nickel and cerium, and the nickel in the solution is reduced to form nano boron nickel.
Embodiment 3
[0029] Embodiment 3: Preparation of nano-amorphous boron-nickel-doped lanthanum hydroxide gel
[0030] Dissolve 1 mole of nickel nitrate and 1 mole of lanthanum nitrate in 1.8 liters of deionized water at a molar ratio of 1:1.5:100 to form a nickel and lanthanum nitrate solution. 0.4 mole of sodium borohydride (15.12 g) was dissolved in 488.88 g of 10 wt % NaOH solution to obtain a lye solution containing 3 wt % sodium borohydride. Sodium borohydride lye is added dropwise to the nitrate solution of nickel and lanthanum, and the nickel in the solution is reduced to form nano-amorphous boron-nickel. Nano-amorphous boron-nickel doped lanthanum hydroxide gel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com