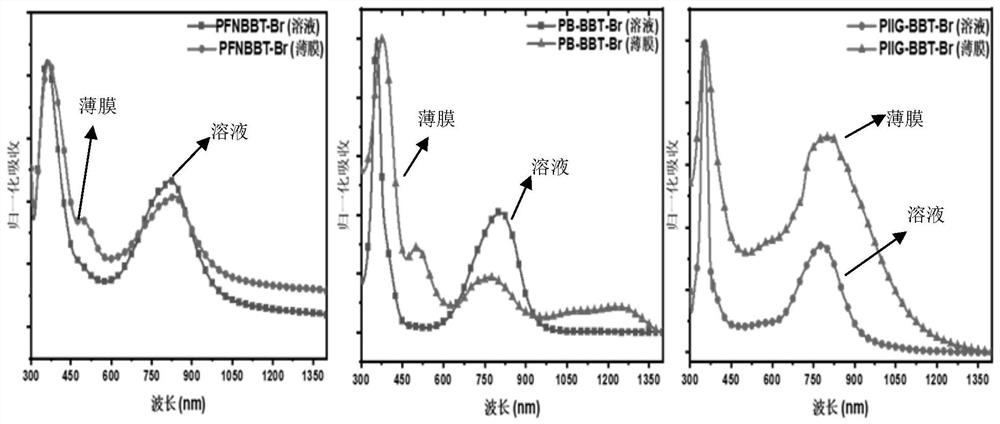
N-type water/alcohol-soluble conjugated polyelectrolyte based on double-free-radical benzobithiadiazole as well as preparation and application of n-type water/alcohol-soluble conjugated polyelectrolyte
A technology of benzobisthiadiazole and conjugated polyelectrolyte, which is applied in the field of n-type water/alcohol-soluble conjugated polyelectrolyte, can solve the problem that it is difficult to effectively realize high-conductivity water/alcohol-soluble conjugated polymers, lack of benzene Problems such as quinone resonance transformation can meet the needs of environmentally friendly chemical processing, improve air stability, and improve transmission efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The chemical reaction equation for the preparation of BBT-Eh:
[0042]
[0043] Monomer 1 was synthesized according to the method reported in the literature (Benzodithiophene–Dithienylbenzothiadiazole Copolymers for Efficient Polymer Solar Cells: Side-Chain Effect on Photovoltaic Performance. ACS Applied Materials & Interfaces, 2018, 10(40), 34355-34362), and monomer 2 was 99% pure reagents were purchased commercially.
[0044] Take monomer 1 (2.45mmol) and monomer 2 (1.1mmol) into a reaction flask with a stirring bar, vacuumize for 5 minutes and pass nitrogen, add 50mL of toluene, and pump for 2 times; add under nitrogen Tetrakis(triphenylphosphine)palladium 36mg, stirred and heated to 100°C, reacted for 24 hours, after the reaction, toluene was removed by a rotary evaporator to obtain a crude product; The solvent was removed, and the crude product was purified by column chromatography to obtain the target compound BBT-EH as a green solid (70% yield). 1 H NMR (500...
Embodiment 2
[0046] The preparation of BBT-Eh-Br, the chemical reaction equation is as follows:
[0047]
[0048] Add BBT-EH (2mmol) dissolved in 25mL tetrahydrofuran in a 50mL round bottom flask with a magnetic stirrer, add N-bromosuccinimide (NBS, 4.05mmol) to the solution under nitrogen, The solution was stirred and reacted at room temperature in the dark for 4 hours, and the tetrahydrofuran was removed by a rotary evaporator, and then the crude product was dissolved in dichloromethane and eluted with a mixed solvent of petroleum ether and dichloromethane (1:1) reagent, the crude product was purified by column chromatography to afford the target compound BBT-EH-Br as a green solid (90% yield). 1 H NMR (500MHz, CDCl 3 ,ppm): 8.80-8.84(s,2H), 2.72-2.64(m,4H), 1.68-1.60(m,2H), 1.42-1.21(m,16H), 1.01-0.82(m,12H).
Embodiment 3
[0050] The preparation of IIG-C6Br, the chemical reaction equation is as follows:
[0051]
[0052] Under the protection of argon, the starting material (E)-6,6'-dibromo-[3,3'-diindolinylidene]-2,2'-dione (10mmol) was dissolved in 150mL ultra-dry N , N-dimethylformamide, add potassium carbonate (25mmol), heat to 85°C and stir for 1 hour, add 1,6-dibromohexane (60mmol), and heat up to 100°C for 24 hours, the reaction The solution was cooled to room temperature, and N,N-dimethylformamide was removed by a rotary evaporator to obtain a viscous crude product; using a mixed solvent of petroleum ether and dichloromethane (1:1) as the eluent, the The crude product was purified to afford the target compound IIG-C6Br as a red needle-like solid (15% yield). 1 H NMR (500MHz, CDCl 3 ,ppm): 9.09-9.05(d,2H),7.20-7.16(dd,2H),6.95-6.92(d,2H),3.80-3.71(t,4H),3.44-3.37(t,4H),1.91 -1.82 (p, 4H), 1.76-1.68 (p, 4H), 1.42-1.37 (m, 4H), 1.32-1.21 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com