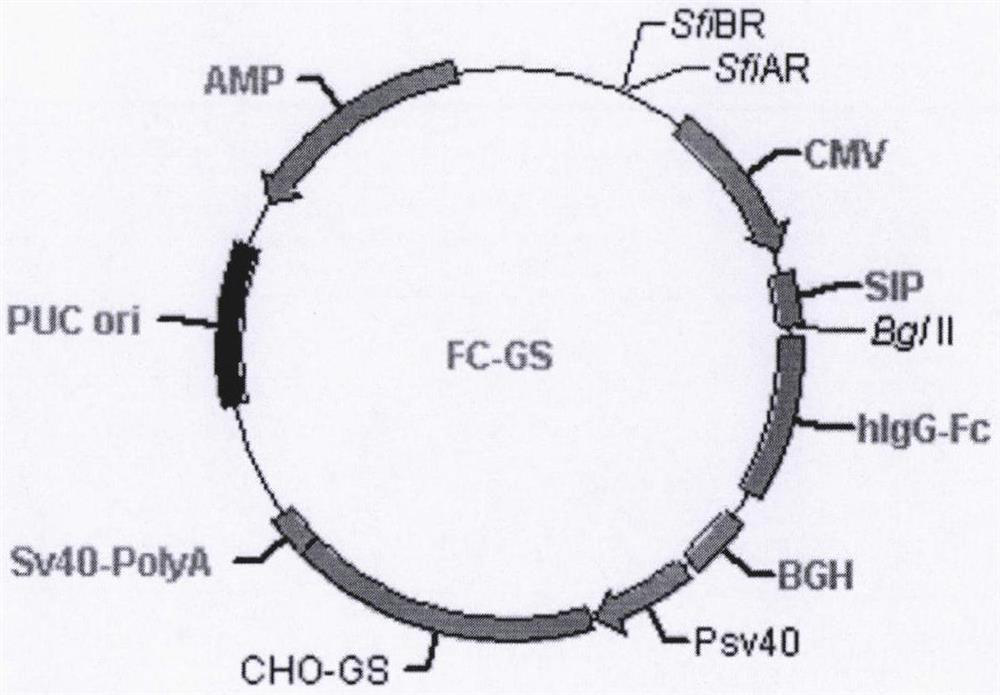
Recombinant human adiponectin expression vector, vector construction method and expression method
A technology of expression vector and construction method, applied in vector, nucleic acid vector, recombinant DNA technology and other directions, can solve the problems of inconsistent evaluation of adiponectin effect and difference of adiponectin effect, and achieves high repeatability, good versatility, and solution. complex effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Embodiment 1 Construction of recombinant human adiponectin expression vector
[0081] Synthesize the cDNA sequence shown in SEQ ID No.1;
[0082] Using the cDNA sequence as a template, it was amplified by PCR. The total reaction system of polymerase chain reaction is: template cDNA 1 μL, forward primer 3 μL, reverse primer 3 μL, 5×PrimeStar Buffer 6 μL, dNTPS (2.5mM each) 0.5 μL, Taq DNA Polymerase 0.5 μL, add ddH 2 0 to 30 μL. The reaction conditions were: pre-denaturation at 95°C for 5 min; denaturation at 95°C for 30 s, annealing at 58°C for 30 s, extension at 72°C for 30 s, 25 cycles; extension at 72°C for 5 min.
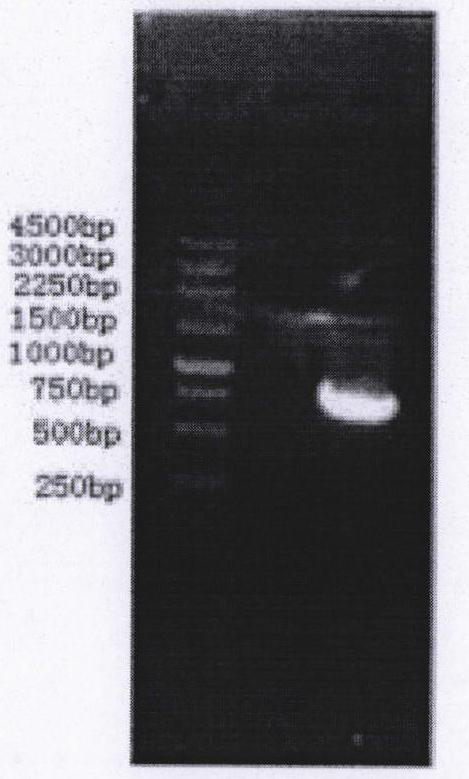
[0083] After the polymerase chain reaction product was analyzed by 10g / L agarose gel electrophoresis, the band at about 748bp was quickly taken for gel tapping. For electrophoresis results see figure 2 , figure 2 There is an obvious band at 748bp, which is the target fragment. The target fragment was recovered by agarose electrophoresis and then d...
Embodiment 2
[0088] Example 2 Expression of recombinant human adiponectin fusion protein in hamster ovary cells
[0089] CRISPR technology knocked out the endogenous glutamine synthetase gene of CHO-K1 cells, took CHO-K1 cells (Invitrogen) in logarithmic growth phase, digested with trypsin, resuspended in PBS solution, and adjusted its density About 1×10 7 cells / ml. The above cell suspension was mixed with 20ug ADIP-FC-GS plasmid, and placed on ice for 5min. Transfer the cell suspension to a pre-cooled electric shock cup (165-2081, Bio-rad, capacity 0.4ml, gap 0.4cm), adjust the electroporator to square wave mode, voltage 280v, time 20ms, electric shock once, total discharge Time 20ms. Transfer the cells to a 10cm culture dish and add 10ml DMEM complete medium. Then place them in a carbon dioxide incubator for overnight cultivation. On the second day, the medium was replaced with a screening medium containing 50 uMMSX (M5379, SIGMA, USA), and the culture was continued for about 14 day...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com