Engineering cyanobacteria for biosynthesis of p-coumaric acid and preparation method of engineering cyanobacteria
A technology for biosynthesis and stearic acid, applied in the field of bioengineering, can solve the problems of long growth cycle, low yield, low yield, etc., and achieve the effects of fast growth, mature genetic manipulation technology, and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: The carrier construction that is used to produce p-myrisin engineering cyanobacteria
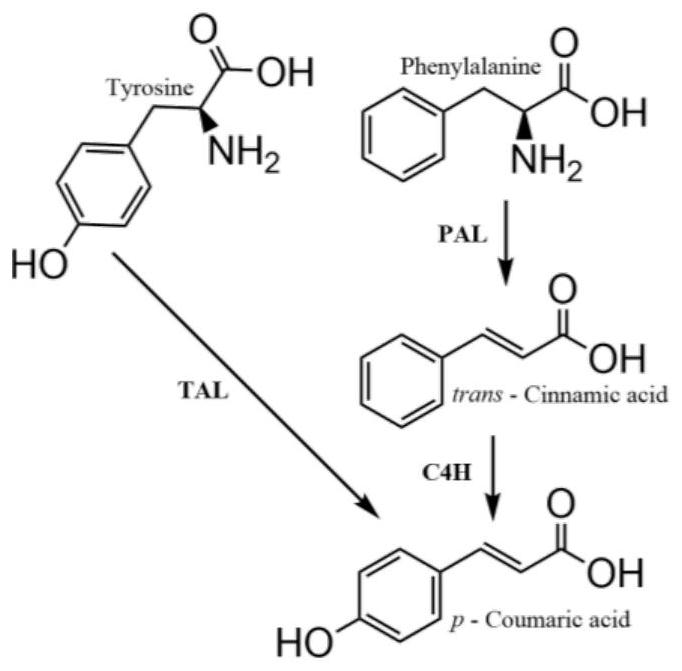
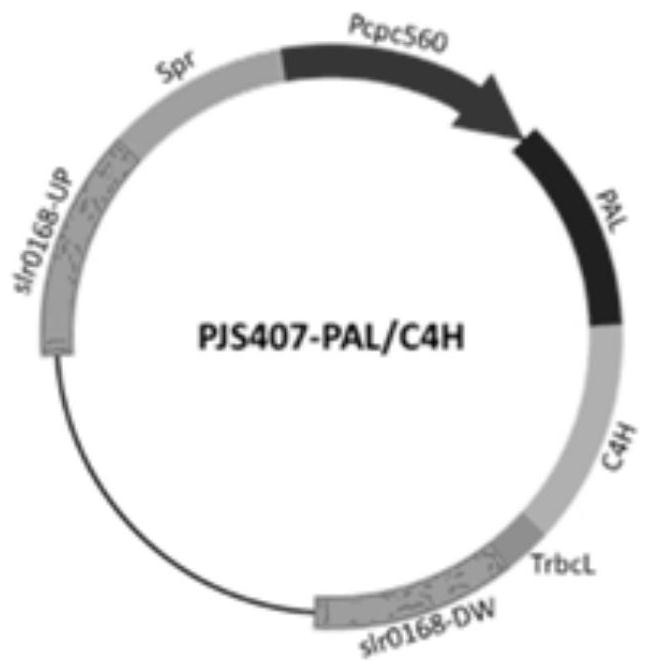
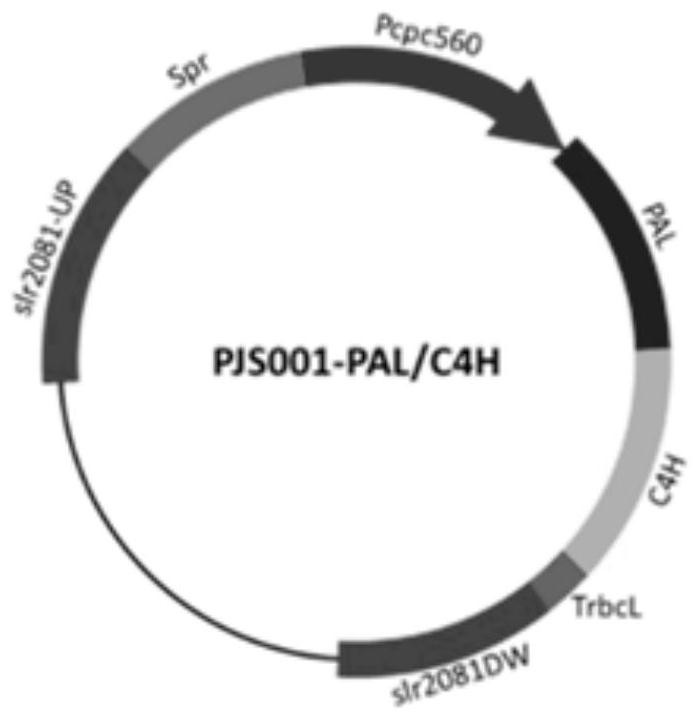
[0048] In order to confirm the role of alaninase and cinnamic acid-4-hydroxylase in the synthesis of p-myrisin in cyanobacteria, and to increase the production of p-myrisin in cyanobacteria by increasing the expression of the enzymes, a The vector pCA0168 used to integrate the phenylalaninase gene (PAL) and the cinnamic acid-4-hydroxylase gene (C4H) driven by the Pcpc560 promoter into the cyanobacterial genome at the slr0168 site. In order to further increase the expression level of alaninase and cinnamic acid-4-hydroxylase so as to improve the output of p-maric acid synthesized by cyanobacteria, a phenylalanase gene (PAL) for driving by the Pcpc560 promoter was constructed. and the cinnamic acid-4-hydroxylase gene (C4H) were integrated into the vector pCA2081 at the slr2081 site of the cyanobacteria genome.
[0049] Above-mentioned carrier transforms cyanobacteria PCC6...
Embodiment 2
[0064] Embodiment 2: Transformation of cyanobacteria and screening of transformants
[0065] (1) Cyanobacterium PCC6803 was cultured statically in BG11 medium under continuous light conditions with a temperature of 30°C and a light intensity of 30 μE m-2s-1. Glucose at a final concentration of 5 mM was added to BG11 during mixed culture. If passaging or screening on a solid plate, add agar powder to a final concentration of 1.4%, TES-NaOH (pH8.0) to 8mM, Na 2 S 2 o 3 to 0.3% and glucose to 5mM.
[0066] (2) Take 10 mL of cyanobacterial cells in the logarithmic growth phase (OD730 is about 0.5-1.0), and collect the cells by centrifugation; wash the cells twice with fresh BG11 medium, and then resuspend the cells in 1 mL of BG11 medium (1.5 g / L NaNO 3 , 40mg / L K 2 HPO 4 ·3H 2 O, 36mg / L CaCl 2 2H 2 O, 6mg / L citric acid, 6mg / L ferric ammonium citrate, 1mg / L EDTA disodium salt, 20mg / L NaCO 3 , 2.9mg / LH 3 BO 3 , 1.8mg / LMnCl 2 4H 2 O,0.22mg / LZnSO 4 ·7H 2 O, 0.39mg / LN...
Embodiment 3
[0075] Embodiment 3: the output of cyanobacteria of genetic engineering transformation
[0076] 1. Experimental procedure: use a transparent conical cell culture flask, fill with 150mL liquid BG11 medium (including the corresponding resistance mentioned above), the initial inoculation concentration is OD730 = 0.1, at 30°C, 30μEm-2s-1 light conditions After culturing under continuous light for one week, when the OD730 reached 1.5, the cell pellet and supernatant were collected by centrifugation, and the cells were broken with a cell disruptor, and used for detection by high performance liquid chromatography.
[0077] 2. Experimental results
[0078] The present inventors respectively detected the production of p-amyric acid in cyanobacteria PCC6803 and SYN005, the production of p-amyric acid was not detected in the wild-type cyanobacteria PCC6803, and the production of p-amyric acid was detected in SYN005. Figure 4 and Figure 5 show the growth of cyanobacteria PCC6803 and SYN0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com