Process for preparing 3-cephem compound
The technology of a compound, cephem, is applied in the field of 3-cephem compound, which can solve the problems of loss, failure to obtain the target object, and the preparation method has not been established, so as to achieve the effect of cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
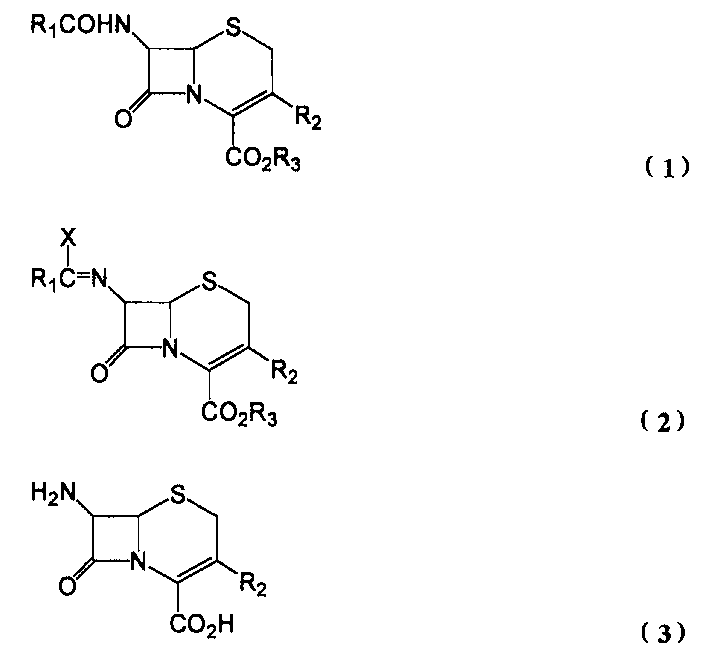
[0037] Weigh PCl in a 1L four-neck flask 5 / pyridine complex 43.9g (1.5eq.), add 250ml of dichloromethane, and cool to 5°C. Add compound (1a) (R 1 = benzyl, R 2 = H, R 3 = benzyl) 50 g, stirred for 1 hour. The reaction solution was cooled to below -10°C, 200 g of m-cresol was added, and stirred at 7-12°C for 5 hours. 150 ml of cold water was added thereto for extraction, and the organic layer was extracted with 150 ml of cold water. The obtained water layer was first treated with 2 g of activated carbon, and then 25% ammonia water was used to adjust the hydrogen ion concentration to pH=4 to precipitate crystals. The precipitated crystals were filtered off and washed with acetone to obtain 18.5 g of the target compound (3a) (90% yield). 1 H NMR (300MHz, DMSO-d 6 / DCl) δ3.60 (dd, J = 18.9, 5.4Hz, 1H), 3.66 (dd, J = 18.9, 3.6Hz, 1H), 5.11 (d, J = 5.4Hz, 1H), 5.16 (d, J =5.4Hz, 1H), 6.53(m, 1H).
Embodiment 2
[0039] Except the PCl in embodiment 1 5 / pyridine complex 43.9g (1.5eq.) into PCl 5 / pyridine complex 32.23g (1.5eq.) and pyridine 12.5ml (1.5eq.), the same reaction as in Example 1 was carried out to obtain the target compound (3a) (R 2 =H) 18.7 g (91% yield). The resulting compound (3a) 1 H NMR is completely consistent with the compound obtained in Example 1.
Embodiment 3
[0041] Except that the temperature after adding 200g m-cresol and time 7~12 ℃, 5 hours become 20~25 ℃, 3 hours, carry out the same reaction of embodiment 1, obtain target compound (3a) as a result (R 2 =H) 18.2 g (yield 88%). The resulting compound (3a) 1 H NMR is completely consistent with the compound obtained in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com