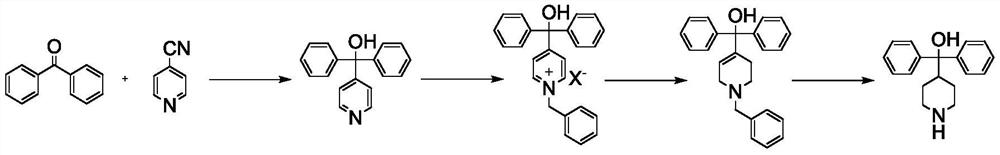
Preparation method of alpha, alpha-diphenyl-4-piperidine methanol
A technology of piperidine methanol and benzyl alcohol, applied in the field of alpha, can solve the problems of unsafe storage and use in industrial production, increased production cost, toxic methyl iodide, etc., and achieves avoiding the problem of three wastes, low production cost, storage and use safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) α, the synthesis of α-diphenyl-4-pyridinemethanol:
[0030] Put 52g (0.5mol) of 4-cyanopyridine, 136.5g (0.75mol) of benzophenone, and 1L of xylene into a 2L four-necked bottle equipped with a nitrogen protection device, and add 25g (1.1mol) of metal sodium particles under nitrogen protection. ), then start heating, reflux reaction for 2 to 3 hours, after the reaction, cool down the system, then add 0.8L of ice water dropwise, a yellow solid precipitates during the dropwise addition, filter, wash the filter cake with clear water, and then reconstitute it with 0.8L of methanol Crystallized to obtain a white solid with a yield of 94%.
[0031] 2) Synthesis of N-benzyl-α, α-diphenyl-4-pyridinemethanol chloride:
[0032] Add 118g (0.45mol) of α,α-diphenyl-4-pyridinemethanol, 63.3g (0.50mol) of benzyl chloride, and 1L of acetonitrile into a 2L four-neck flask, then start heating, reflux for 2-3 hours, and the reaction ends Afterwards, the system was cooled down, filtere...
Embodiment 2
[0038] 1) α, the synthesis of α-diphenyl-4-pyridinemethanol:
[0039] Put 52g (0.5mol) of 4-cyanopyridine, 91g (0.5mol) of benzophenone, and 1L of xylene into a 2L four-necked bottle equipped with a nitrogen protection device, and add 17.3g (0.75mol) of metal sodium particles under nitrogen protection. ), then start heating, reflux reaction for 2 to 3 hours, after the reaction, cool down the system, then add 0.8L of ice water dropwise, a yellow solid precipitates during the dropwise addition, filter, wash the filter cake with clear water, and then reconstitute it with 0.8L of methanol Crystallized to obtain a white solid with a yield of 92%.
[0040] 2) Synthesis of N-benzyl-α, α-diphenyl-4-pyridinemethanol bromide:
[0041] Add 118g (0.45mol) of α,α-diphenyl-4-pyridinemethanol, 77g (0.45mol) of benzyl bromide, and 1L of acetonitrile into a 2L four-neck flask, then start heating, and reflux for 2 to 3 hours. The system was cooled down, filtered, and the filter cake and 0.6 L...
Embodiment 3
[0047] 1) α, the synthesis of α-diphenyl-4-pyridinemethanol:
[0048] Put 52g (0.5mol) of 4-cyanopyridine, 182g (1mol) of benzophenone, and 1L of xylene into a 2L four-necked bottle equipped with a nitrogen protection device, and add 8.7g (1.25mol) of lithium metal under nitrogen protection. Then start heating, reflux reaction for 2 to 3 hours, after the reaction, cool down the system, then add 0.8L of ice water dropwise, during the dropwise addition, a yellow solid precipitates, filter, wash the filter cake with water, and then recrystallize with 0.8L of methanol, A white solid was obtained with a yield of 96%.
[0049] 2) Synthesis of N-benzyl-α, α-diphenyl-4-pyridinemethanol chloride:
[0050] Add 118g (0.45mol) of α,α-diphenyl-4-pyridinemethanol, 68.4g (0.54mol) of benzyl chloride, and 1L of acetonitrile into a 2L four-neck flask, then start heating, reflux for 2-3 hours, and the reaction ends Afterwards, the system was cooled down, filtered, and the filter cake and 0.6L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com