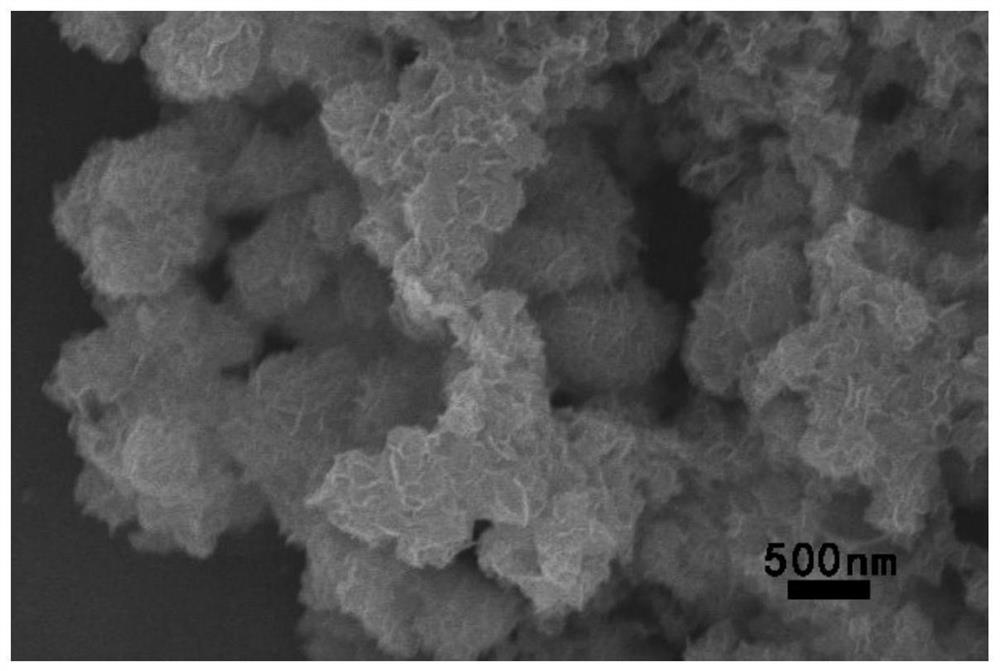
Heterostructure catalyst for decomposing water into hydrogen by using solar energy and preparation method of catalyst
A heterogeneous structure and catalyst technology, applied in chemical instruments and methods, physical/chemical process catalysts, hydrogen production, etc., can solve problems such as poor carrier mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The preparation method of the heterostructure catalytic material comprises the following steps:
[0044] Step 1. Preparation of SiO 2 , urea, nickel salt, deionized water mixed solution; the SiO 2 Disperse nanospheres into deionized water, then add urea and nickel salt and mix evenly to prepare mixed solution 1;
[0045] Step 2, transfer the mixed solution to a hydrothermal reaction kettle, and react at 105°C for 12h;
[0046] Step 3. After natural cooling, the product was washed with deionized water, centrifuged, and dried at 60°C to obtain SiO 2 @nickel silicate powder;
[0047] Step 4. Preparation of SiO 2 @nickel silicate, sodium sulfide, deionized water mixed solution; SiO 2 @nickelsilicate is dispersed in deionized water, adding sodium sulfide, and using NaOH solution to adjust the pH value of the solution to obtain mixed solution 2;
[0048] Step 5, transfer the mixed solution 2 to a hydrothermal reaction kettle, and react at 160°C for 12-20h;
[0049] Ste...
Embodiment 1
[0057] Example 1: 3.33 mmol SiO 2 , 16.65mmol urea and 0.557mmol Ni(NO 3 ) 2 .6H 2 O was dispersed in 40ml deionized water;
[0058] After mixing evenly, transfer to a hydrothermal reaction kettle and react at 105°C for 12 hours;
[0059] After natural cooling, the product was washed with deionized water-centrifuged 5 times, and dried at 60 °C to obtain SiO 2 @nickelsilicate powder;
[0060] 0.1g SiO 2 @nickel silicate dispersed in 40ml deionized water, add 1.29mmol Na 2 S·9H 2 O, fully mixed;
[0061] Use NaOH solution to adjust the pH of the solution to 13.4, then transfer to a hydrothermal reaction kettle, and react at 160°C for 18h;
[0062] After natural cooling, wash and centrifuge with deionized water for 4 times to collect the black precipitate, and vacuum dry at 60°C to obtain NiS hollow nanosphere powder;
[0063] Dissolve 0.474mmol NiS and 12mL glycerin in 40ml water and stir well;
[0064] Then add 1.6mmol ZnCl 2 , 1.6mmol InCl 3 4H 2 O and 3.2mmol TA...
Embodiment 2
[0074] Example 2: 13.3 mmol SiO 2 , 66.6mmol urea and 2mmol Ni(NO 3 ) 2 .6H 2 O was dispersed in 160ml deionized water;
[0075] After mixing evenly, transfer to a hydrothermal reaction kettle and react at 105°C for 12 hours;
[0076] After natural cooling, the product was washed with deionized water-centrifuged 5 times, and dried at 60 °C to obtain SiO 2 @nickelsilicate powder;
[0077] 0.2g SiO 2 @nickel silicate dispersed in 80ml deionized water, add 2.581mmol Na 2 S·9H 2 O, fully mixed;
[0078] Use NaOH solution to adjust the pH of the solution to 13.4, then transfer to a hydrothermal reaction kettle, and react at 160°C for 16h;
[0079] After natural cooling, wash and centrifuge with deionized water for 4 times to collect the black precipitate, and vacuum dry at 60°C to obtain NiS hollow nanosphere powder;
[0080] Dissolve 0.095mmol NiS and 2mL glycerin in water and stir well;
[0081] Then add 0.399mmol ZnCl 2 , 0.411mmol InCl 3 4H 2 O and 0.799mmol TAA; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com