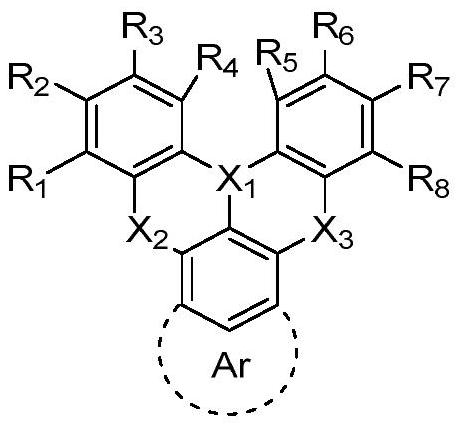
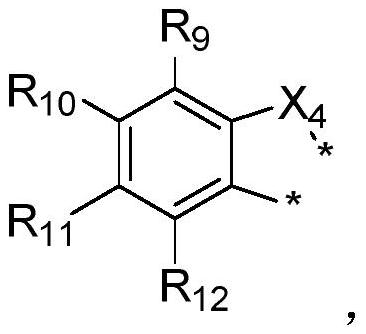
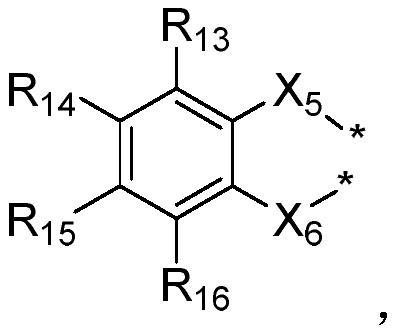
Boron-based organic electroluminescent material as well as preparation method and application thereof
A luminescent and electromechanical technology, applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056]
[0057] material c
[0058] Step 1: In a 100mL three-necked flask, add 2,4-difluoro-7-phenyldibenzofuran (5.61g, 20mmol), phenol (4.71g, 50mmol), potassium carbonate (40mmol), N-methyl Pyrrolidone 60mL, in a nitrogen atmosphere, heat up to 170°C, monitor the completion of the reaction in the liquid phase, cool to room temperature, add water, filter, and reflux the filter cake with 3 times of ethanol to obtain the above material c5.14g, yield 60 %;
[0059] Step 2: In a 100 mL three-neck flask, slowly add 2.5M n-hexane tert-butyllithium solution (32.5 mL, 13 mmol) into the above material c (5.61 g, 10 mmol) in tert-butylbenzene solution (50 mL). Cool down to -40°C, add boron tribromide (1.42mL, 15mmol) slowly, rise to room temperature and stir for 1.5h, cool down to 0°C, add N,N-diisopropylethylamine (1.29g, 10mmol), heat up Stir at 120°C for 4h, cool to room temperature, add acetic acid to quench the reaction, filter, filter cake is eluted with toluene, the organi...
Embodiment 2
[0062]
[0063] material a intermediate c
[0064] Step 1: In a 250mL three-necked flask, add 7-chloro-2,4-difluorodibenzofuran (8.35g, 35mmol), carbazole (5.85g, 35mmol), potassium carbonate (9.67g, 70mmol), di Toluene 100mL, under a nitrogen atmosphere, add cuprous iodide (0.76g, 4mmol) and 1,10-phenanthroline (1.44g, 8mmol), heat up to 125 ° C, the liquid phase monitors the completion of the reaction, cool to room temperature, add Water and dichloromethane were separated, the organic phase was concentrated, and the crude product was beaten with 5 times of ethanol under reflux to obtain the above-mentioned material a9.82g, with a yield of 76%;
[0065] Step 2: Replace 2,4-difluoro-7-phenyldibenzofuran in Example 1 with 7.39 g of the material a of this example, and the other processes are the same as Step 1 of Example 1 to obtain the above material c 5.90g, yield 57%;
[0066] Step 3: Replace the material c in Example 1 with 5.17 g of the material c in this example, and ...
Embodiment 3
[0069]
[0070] material c
[0071] Step 1: Replace 2,4-difluoro-7-phenyldibenzofuran in Example 1 with 4.08g of 2,4-difluorodibenzofuran, and replace phenol with 4-(9H-carbazole- 9-base) phenol 12.96g, other process can obtain above-mentioned material c6.96g with the step one of embodiment 1, yield 51%;
[0072] Step 2: Replace material c in Example 1 with 6.83 g of material c in this example, and the other process is the same as step 2 in Example 1 to obtain 2.07 g of the target compound (2-13), with a yield of 30%;
[0073] The obtained compound was identified by LC-MS and elemental analyzer: [M+1], 690.35; C48H27N2, C 83.50, H3.94, N4.05.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com