Method for preparing (S)-3-butyne-2-propynylamine through biological catalysis
A technology of biocatalysis and propargylamine, applied in the field of biocatalytic chemical reactions, can solve the problems of low selectivity, cumbersome hydrogenation steps, and low conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
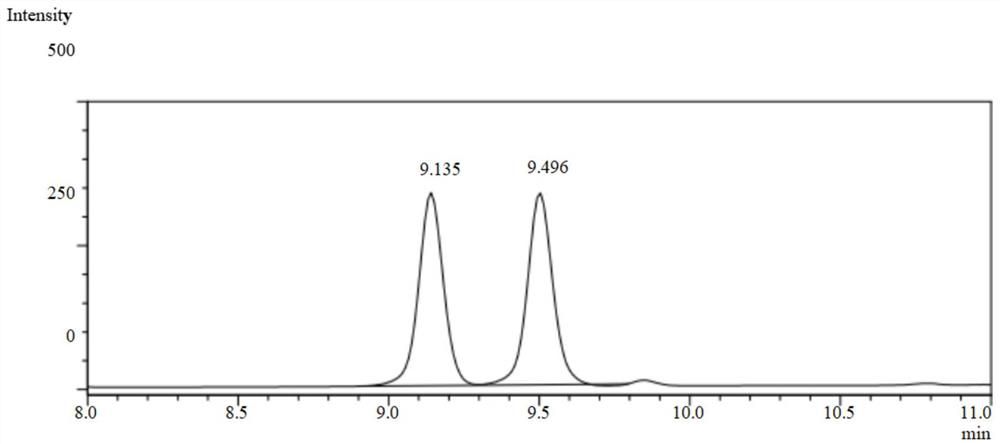
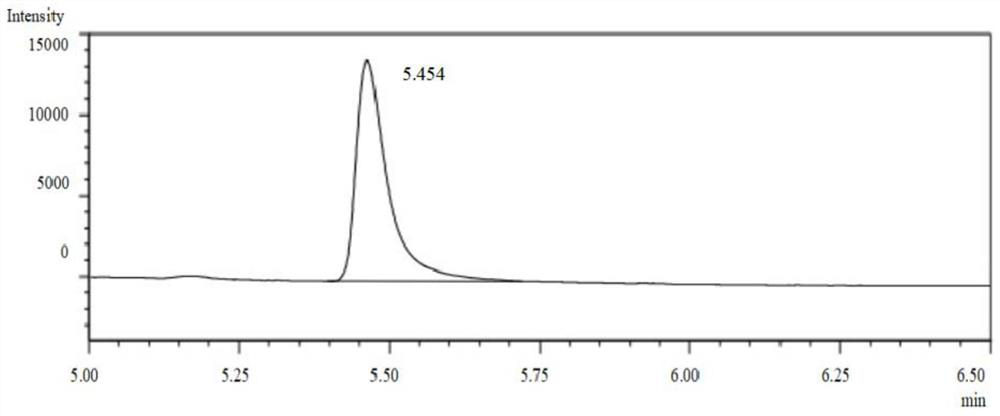
[0054] In an 8 mL glass reaction vial, add 4 mL of ammonium chloride buffer. Add a small amount of ammonia water several times, and keep stirring with a glass rod. After the reaction solution is uniform, submerge the electrode into the reaction solution, measure the pH value of the reaction solution again, adjust the pH value to 8.5, shake the glass reaction bottle gently, and wait after the electrode is submerged in the reaction solution. After the number is stable, read it, measure it three times in parallel, and record it, and finally take the average value of the three measurements, and the pH value of the reaction solution is 8.5. After the measurement, clean the electrode with distilled water, blot the distilled water dry with filter paper, and put on the composite electrode cover, and the measurement step is completed. Then add 50mg of imine reductase lyophilized powder and 1mg of nicotinamide adenine dinucleotide phosphate (oxidized state) (NADP), 5mg of glucose dehydr...
Embodiment 2
[0060] First, add 21.40 g of ammonium chloride into 1 L of pure water, stir well until the solid is clear, and prepare 400 mmol of ammonium chloride buffer solution. The buffer concentration was measured with a pH meter, and the initial pH value was 7.5. In an 8ml glass reaction vial, add 4ml of ammonium chloride buffer. Ammonia water was added to adjust the pH value so that the reaction solution in the 8ml glass reaction bottle reached 8.5. Then add 50mg of imine reductase (AoIRED) lyophilized powder and 1mg of nicotinamide adenine dinucleotide phosphate (oxidized state) (NADP), 5mg of glucose dehydrogenase (GDH), and 100mg of glucose to the reaction solution and stir well Then 20 mg of the substrate acetylenone was added. Put it into a full-temperature constant temperature culture shaker, adjust and control the reaction temperature to 30°C, and continuously stir at a constant temperature for 24 hours to make it fully react.
[0061] Take 1ml of the reaction solution and a...
Embodiment 3
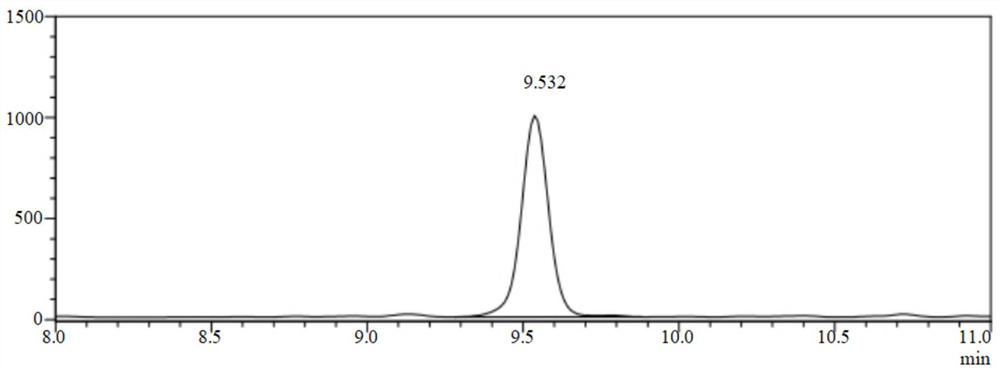
[0064] First, add 21.40 g of ammonium chloride into 1 L of pure water, stir well until the solid is clear, and prepare 400 mmol of ammonium chloride buffer solution. The buffer concentration was measured with a pH meter, and the initial pH value was 7.5. In an 8ml glass reaction vial, add 4ml of ammonium chloride buffer. Ammonia water was added to adjust the pH value so that the reaction solution in the 8ml glass reaction bottle reached 10. Then add 50mg of imine reductase (AoIRED) lyophilized powder and 1mg of nicotinamide adenine dinucleotide phosphate (oxidized state) (NADP), 5mg of glucose dehydrogenase (GDH), and 100mg of glucose to the reaction solution and stir well Then 20 mg of the substrate acetylenone was added. Put it into a full-temperature constant temperature culture shaker, adjust and control the reaction temperature to 30°C, and continuously stir at a constant temperature for 24 hours to make it fully react.
[0065] Take 1ml of the reaction solution and ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com