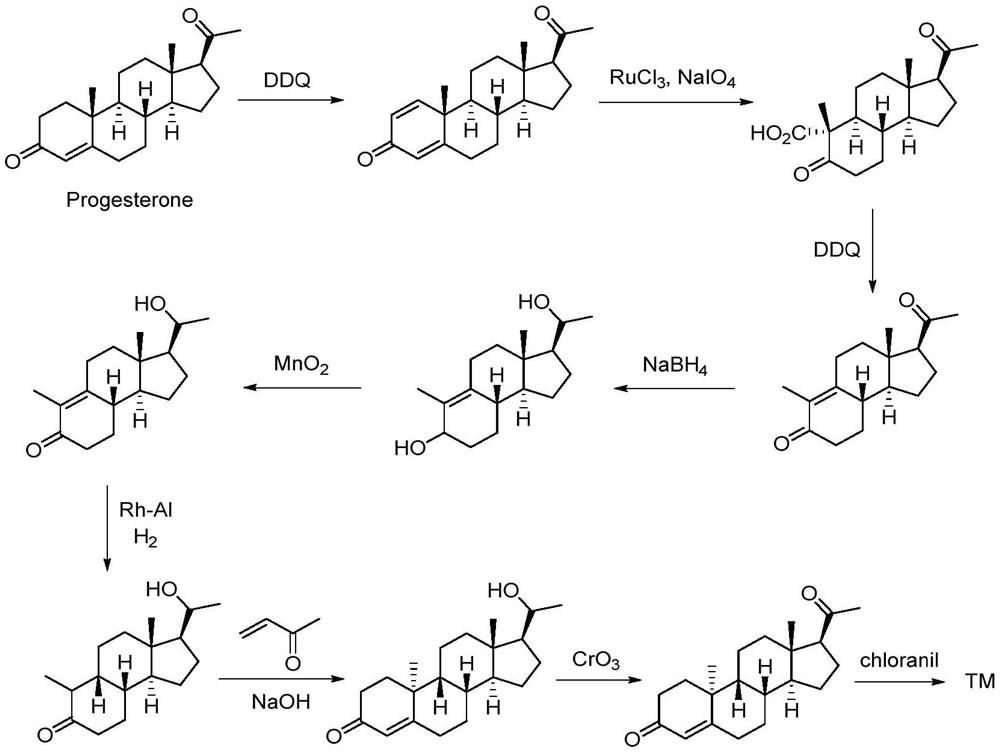
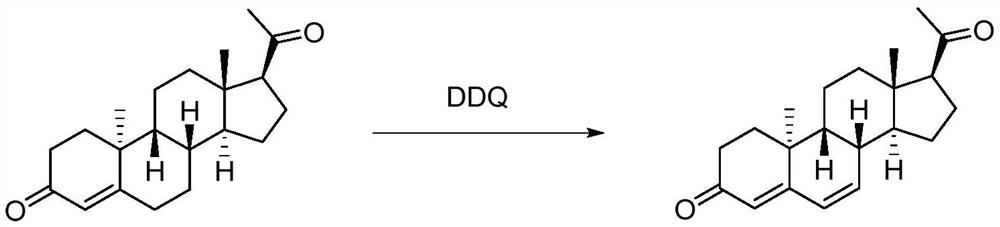
Method for synthesizing dydrogesterone
A technology of dydrogesterone and a synthesis method is applied in the field of production technology of steroid drugs, and can solve the problems of environmental pollution, hidden safety hazards, three wastes discharge and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
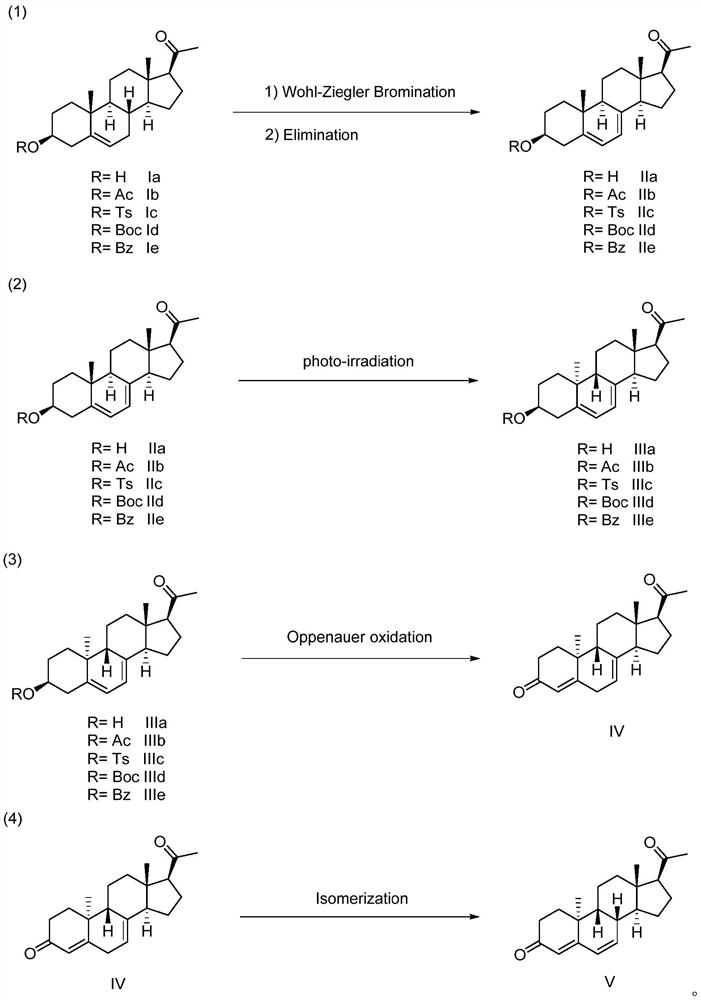
[0024] Add 35.8 g of pregnenolone acetate (Ib), 28.4 g of dibromohydantoin and 3.28 g of azobisisobutyronitrile to 1 L of petroleum ether (60-90° C.), heat the mixture under reflux for 0.5 to 1 hour, and cool to After room temperature, filter and wash with chloroform (3X100mL). After combining the filtrates, the solvent was recovered by distillation under reduced pressure. Add 200 mL of 2,4,6-collidine to the distillation residue, heat at 140°C for 15 minutes under nitrogen protection, and cool to room temperature. Add 100mL of petroleum ether, wash with water three times (3×100mL), then wash with 2N sulfuric acid three times (3×100mL), finally wash with 5% sodium carbonate, wash with water, combine the organic phases, and dry over anhydrous sodium sulfate. After filtration, the residue after distilling off the solvent was recrystallized with methanol / acetone (v / v=150mL / 10mL) to obtain 26.7 g of IIb (75%, purity 99.8%).
Embodiment 2
[0026] NBS, N-bromoacetamide, bromochlorohydantoin, N-chlorosuccinimide (NCS), dichlorohydantoin (DCDMH), trichloroisocyanuric acid, N-iodosuccinyl Imine (NIS), diiodohydantoin (DIDMH) instead of dibromohydantoin, others are the same as in Example 1, and the productive rate of compound IIb is respectively 76%, 60%, 66%, 70%, 55%, 61 %, 58%, 59%.
Embodiment 3
[0028] Use azobisisoheptanonitrile, di-tert-butyl azodicarboxylate, diisophenylpropyl azodicarboxylate, di-tert-butoxy azide, tert-butanol peroxide (TBHP), tert-butyl peroxyether (DTBP), benzoyl peroxide (BPO), dicumyl peroxide (DCP), dilauroyl peroxide (DLP), tert-butyl peroxy acetate (TBPA), m-chloroperbenzoic acid (mCPBA) Instead of AIBN, the others were the same as in Example 1, and the yields of compound IIb were 70%, 68%, 72%, 50%, 52%, 65%, 53%, 49%, 55%, and 43%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com