Detection method of azithromycin related substances
A technology of azithromycin and detection method, applied in the field of drug detection, which can solve the problems of inability to separate and measure known impurities and inability to carry out strict quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
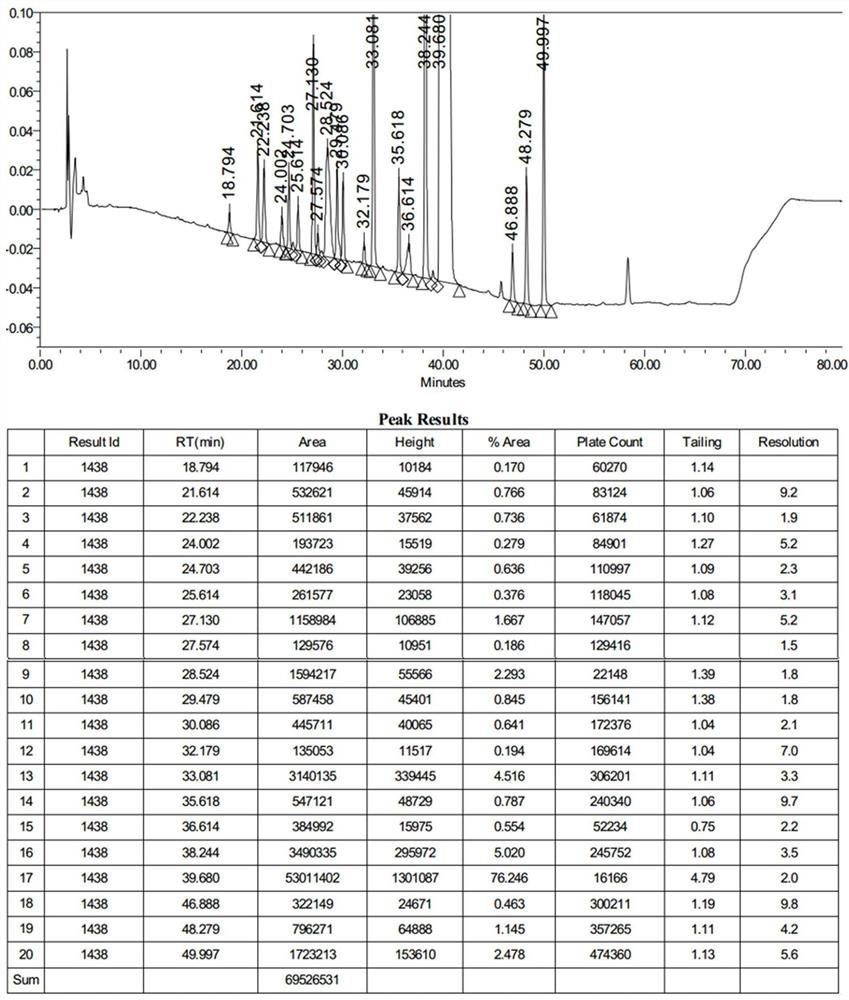
[0067] This embodiment provides a method for the determination of related substances of azithromycin by high performance liquid chromatography, which is determined by the method of chromatographic conditions:
[0068] Chromatographic column: use octadecylsilane bonded silica gel as filler, choose Waters ShieldRP18, 4.6mm×250mm, 3.5μm chromatographic column;
[0069] Mobile phase A: 0.05mol / L dipotassium hydrogen phosphate solution (adjust the pH value to 8.20-8.25 with 20% phosphoric acid solution)-acetonitrile (98:2);
[0070] Mobile phase B: acetonitrile-methanol (90:10);
[0071] Column temperature: 45~48℃;
[0072] Detection wavelength: 210nm; injection volume: 50μL
[0073] Flow rate: 0.8mL / min;
[0074] Solvent: Take 1.73g of ammonium dihydrogen phosphate, add water to dissolve and dilute to 1000ml, adjust the pH value to 10.0 with ammonia test solution)-methanol-acetonitrile (35:35:30);
[0075] Gradient elution was used.
[0076] The program of gradient elution ...
experiment example 1
[0086] Experimental Example 1 System Suitability Test
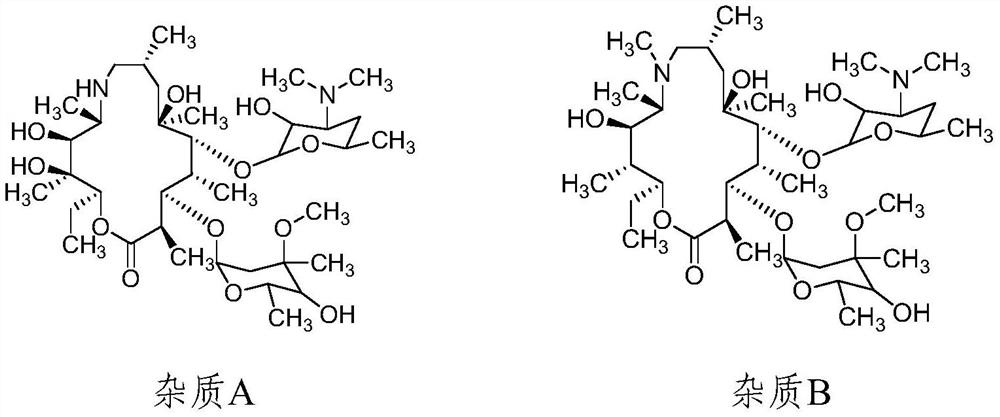
[0087] Preparation of each impurity positioning solution: Accurately weigh azithromycin impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, impurity G, impurity H, impurity I, impurity J, impurity K, impurity L, impurity M, Impurity N, Impurity O, Impurity Q 1 , impurity Q 2 19 impurities such as impurity R, impurity S, and the appropriate amount of azithromycin reference substance, add solvent respectively (ammonium dihydrogen phosphate 1.73g, add water to dissolve and dilute to 1000ml, adjust the pH value to 10.0 with ammonia test solution)-methanol-acetonitrile ( 35:35:30)) dissolved and diluted to make G, D, K, Q EP (Q 2 ) four known impurities are all 20μg / mL, other impurities A, F, J, N, O, P, Q (Q 1 ), the concentrations of S, C, E, H, I, L, M, and R are all 50 μg / mL, and the concentration of the azithromycin is 10 mg.
[0088]Preparation of the test solution: Take about 100 mg of azithromy...
experiment example 2
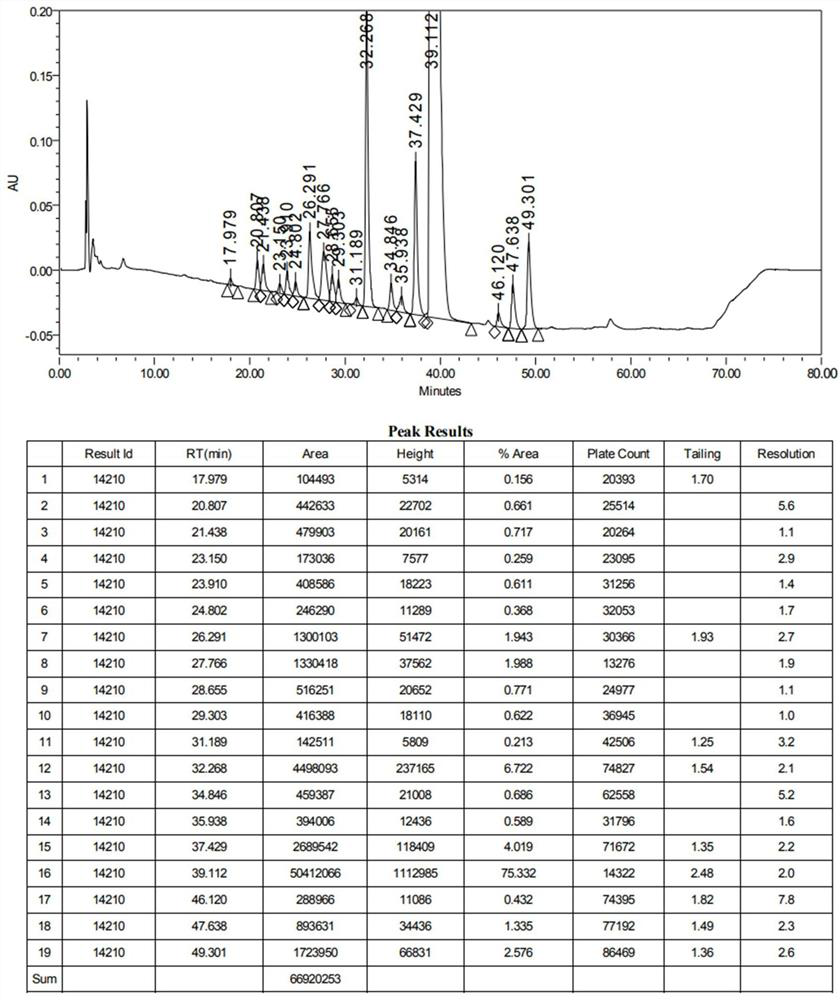
[0110] Experimental example 2 linearity and range test
[0111] Solvent: (1.73g of ammonium dihydrogen phosphate, dissolved in water and diluted to 1000ml, adjusted to pH 10.0 with ammonia test solution)-methanol-acetonitrile (35:15:50)).
[0112] Linear sample solution: Accurately weigh azithromycin impurity A, impurity B, impurity C, impurity D, impurity E, impurity F, impurity G, impurity H, impurity I, impurity J, impurity K, impurity L, impurity M, impurity N, Impurity O, Impurity Q 1 , impurity Q 2 , impurity R, impurity S and azithromycin reference substance in appropriate amounts, respectively add solvent to dissolve to make each stock solution, accurately measure appropriate amount, dilute with solvent to form a series of linear sample solutions, shake well, and obtain.
[0113] Accurately measure 50 μL of each of the above solutions, inject them into a liquid chromatograph, and record the chromatograms. The results are shown in Table 3.
[0114] Table 3 Linearity...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Volume fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com