Tumor photodynamic therapy series drugs and application thereof
A photodynamic therapy and photodynamic therapy technology, applied in antitumor drugs, drug combinations, nano-drugs, etc., can solve the problems of easy recurrence of disease, enhanced toxic and side effects, and hinder drug delivery, so as to improve tumor extracellular matrix, reduce Compression of blood vessels and reduction of solid stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] In some embodiments, the preparation method of the drug B used in the present invention includes the following steps:
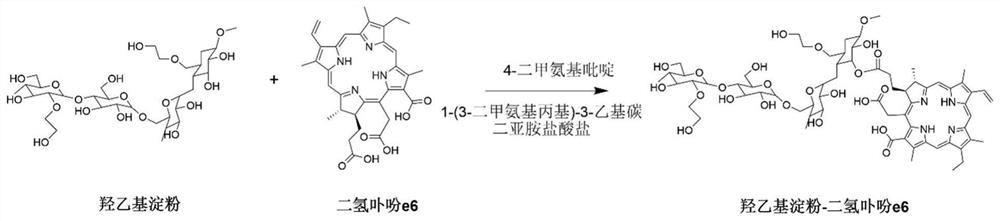
[0057] (1) Mix the organic solution of chlorin e6, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and 4-dimethylaminopyridine, stir at room temperature for 2~ After 4 hours, a solution of chlorin e6 activated at the carboxyl end was obtained;
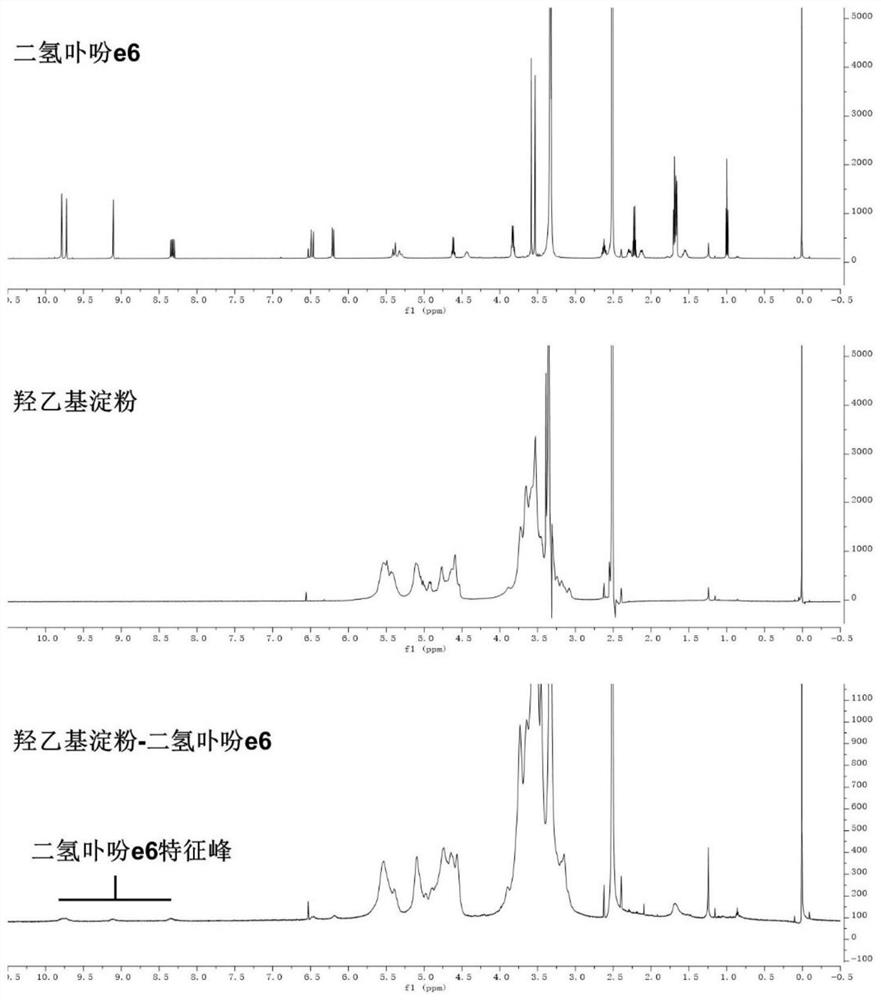
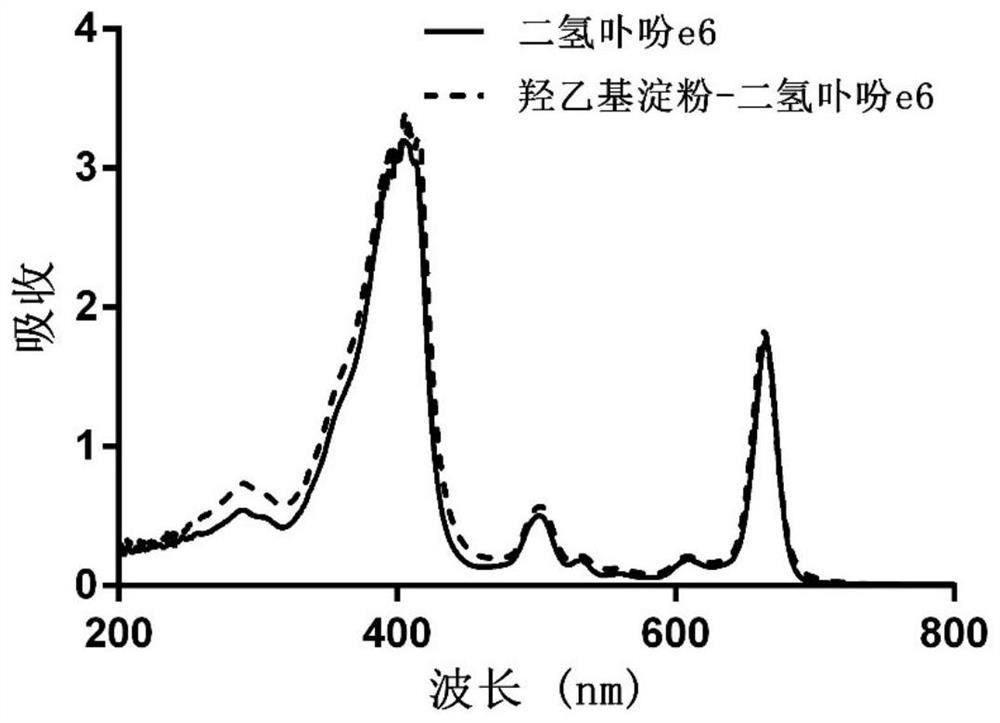
[0058] (2) Mix hydroxyethyl starch with the carboxy-terminally activated chlorin e6 solution described in step (1), stir and react at 20-60°C for 12-72 hours, and undergo esterification to obtain hydroxyethyl starch A mixture of starch-grafted chlorin e6;
[0059] (3) adding an organic solvent to the mixture described in step (2) to precipitate the hydroxyethyl starch grafted chlorin e6 product, and washing the solid precipitate obtained by centrifugation with the organic solvent for 2 to 3 times; After the obtained precipitate is dissolved in ultrapure water, dialyze in ultrapure water with a dia...
Embodiment 1
[0074] A conjugate of hydroxyethyl starch grafted chlorin e6, its preparation method comprises the steps:
[0075] (1) Activate the carboxyl group of chlorin e6: weigh chlorin e6 (33.12 mg), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (21.25 mg ) and 4-dimethylaminopyridine (6.79 mg) in a 25 mL single-necked round bottom flask, 10 mL of anhydrous dimethyl sulfoxide was added thereto, and stirred at room temperature for 2 hours to obtain a solution of carboxy-terminally activated chlorin e6.
[0076] (2) Esterification reaction: Weigh dry hydroxyethyl starch (HES 130 / 0.4, 150 mg), dissolve it in 5 mL of anhydrous dimethyl sulfoxide, and add it to the carboxy-terminally activated disulfide described in step (1). In the hydroporphin e6 solution, the reaction was stirred at 40°C for 48h.
[0077] (3) Purification: the mixture described in step (2) was added dropwise into 200 mL of dichloromethane to precipitate the hydroxyethyl starch grafted chlorin e6 product. ...
Embodiment 2
[0079] A conjugate of hydroxyethyl starch grafted chlorin e6, its preparation method comprises the steps:
[0080] (1) Activate the carboxyl group of chlorin e6: weigh chlorin e6 (66.24 mg), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (42.50 mg ) and 4-dimethylaminopyridine (13.58 mg) in a 25 mL single-necked round bottom flask, 10 mL of anhydrous dimethyl sulfoxide was added thereto, and stirred at room temperature for 2 hours to obtain a carboxy-terminally activated chlorin e6 solution.
[0081] (2) Esterification reaction: Weigh dry hydroxyethyl starch (HES 40 / 0.5, 300 mg), dissolve it in 5 mL of anhydrous dimethyl sulfoxide, and add it to the carboxy-terminally activated diethyl starch described in step (1). In the hydroporphin e6 solution, the reaction was stirred at 30°C for 48h.
[0082] (3) Purification: the mixture described in step (2) was added dropwise into 200 mL of dichloromethane to precipitate the hydroxyethyl starch grafted chlorin e6 product....
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com