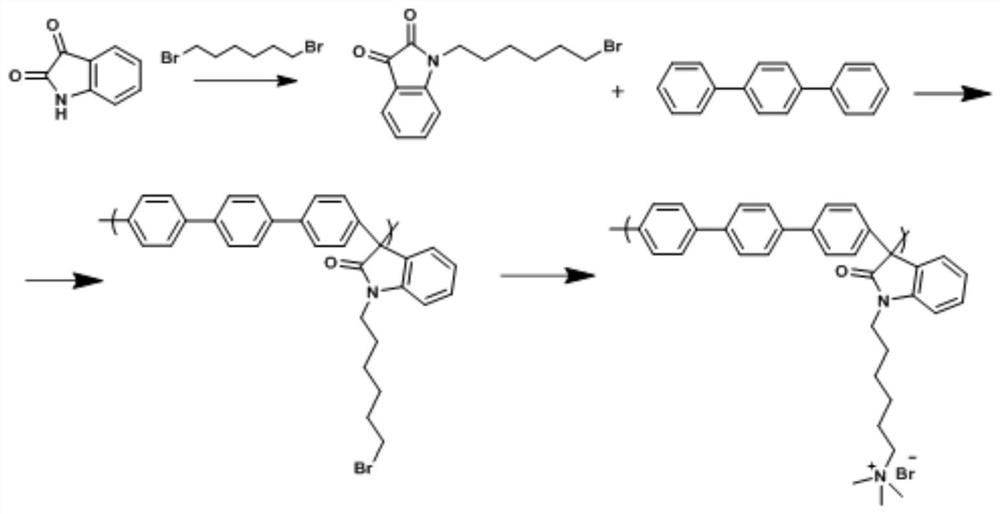
Long side chain type polyarene isatin alkaline membrane for fuel cell and preparation method of long side chain type polyarene isatin alkaline membrane
A fuel cell and polyaromatic technology, applied in fuel cells, circuits, electrical components, etc., can solve the problems of limited alkali resistance and ion conductivity, poor alkali resistance, poor mechanical properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: the synthesis of poly-terphenyl-bromohexyl isatin basic film
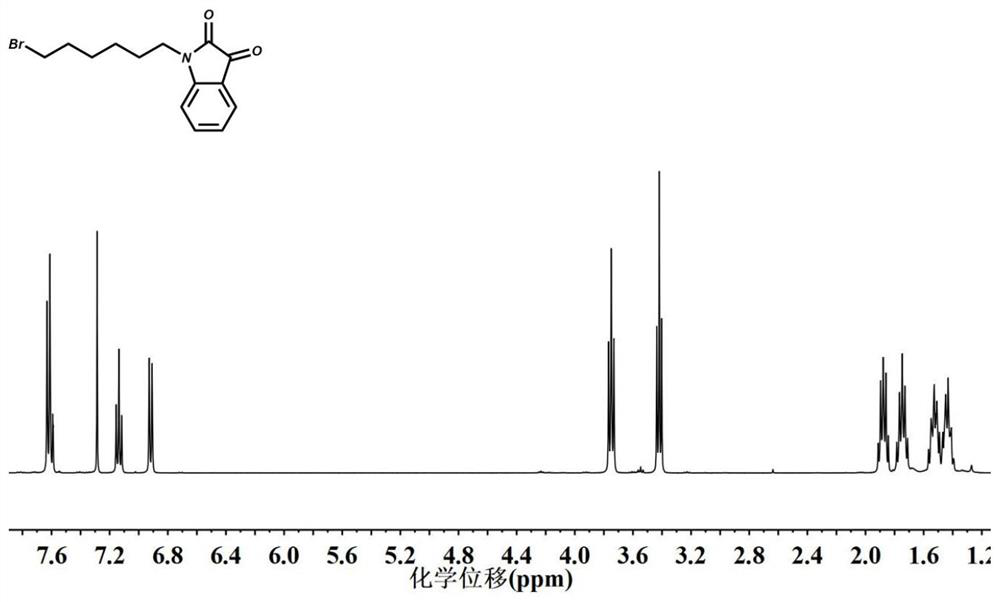
[0048] (1) Synthesis of 1-(6-bromohexyl)-indoline-2,3-dione
[0049] Dissolve 5 g of isatin in 200 mL of N,N-dimethylformamide (DMF), and add the solution to 100 mL of DMF suspension containing 1.23 g of NaH at 0°C, stir for 20 minutes, and add 5.5 mL of 1,6-dibromohexane, the reaction mixture was heated at 60°C for 4 hours, after the reaction was completed, 50 mL of deionized water was added to quench the reaction, the mixture was extracted three times with ethyl acetate, and the organic layer was washed with anhydrous sulfuric acid Sodium was dried for 8 hours, and after the organic solvent was evaporated by rotary evaporation, a brown-red product was obtained. The product was further purified by column chromatography using n-hexane:ethyl acetate (10:3), and the organic solution was rotary evaporated to obtain the product 1-(6 -Bromohexyl)-indoline-2,3-dione (bromohexyl isatin);
[0050] (2...
Embodiment 2
[0058] Embodiment 2: the preparation of polybiphenyl-bromhexyl isatin basic film
[0059] (1) Synthesis of 1-(6-bromohexyl)-indoline-2,3-dione
[0060] With embodiment 1.
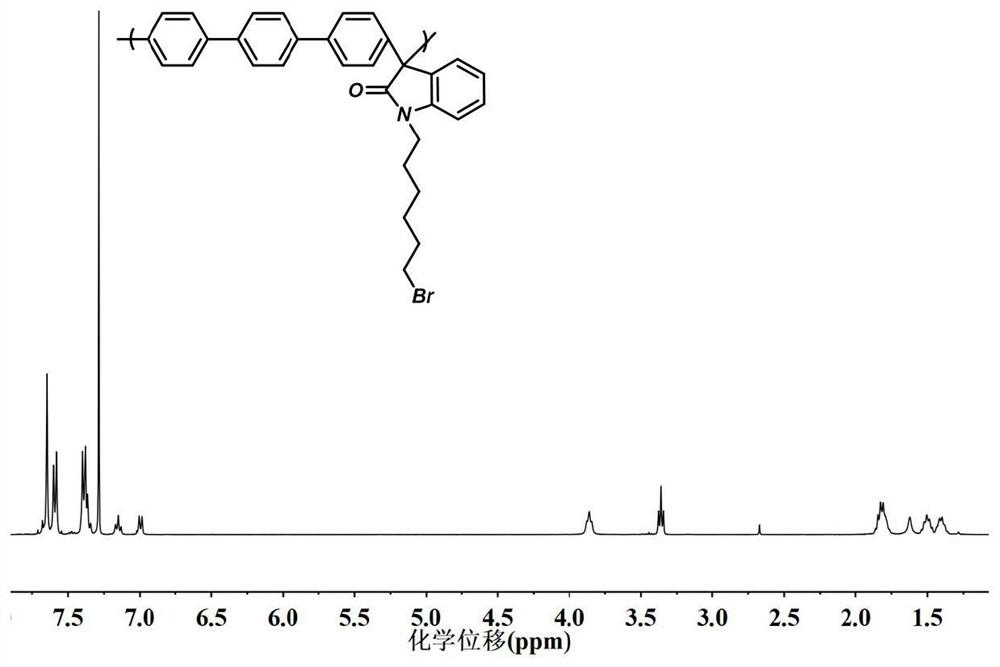
[0061] (2) Synthesis of polybiphenyl-bromohexyl isatin main chain
[0062] 1-(6-bromohexyl)-indoline-2,3-diketone (bromohexyl isatin) prepared by 2g biphenyl and 3.48g of step (1) were dissolved in 10mL of dichloromethane, in Add 9.6mL of trifluoroacetic acid and 11.5mL of trifluoromethanesulfonic acid in an ice bath, and stir and react at room temperature for 4 hours. After the reaction, a viscous dark green solution is obtained, which is poured into methanol to obtain a white fibrous product , the product was filtered, washed three to five times with deionized water until neutral, to obtain polybiphenyl-bromohexyl isatin polyelectrolyte, which was dried for use;
[0063] (3) Synthesis of quaternized polybiphenyl-bromohexyl isatin
[0064] 2 g of quaternized polybiphenyl-bromohexyl isatin prepared in s...
Embodiment 3
[0069] Embodiment 3: the synthesis of poly-terphenyl-bromopropyl isatin basic film
[0070] (1) Synthesis of 1-(3-bromopropyl)-indoline-2,3-dione
[0071] Dissolve 5g of isatin in 200mL of N,N-dimethylformamide (DMF), and add the solution to 100mL of DMF suspension containing 1.23gNaH at 0°C, stir for 20 minutes, add 3.47mL of 1,3-dibromopropane, the mixture was stirred and reacted at 60°C for 4 hours. After the reaction was completed, 50 mL of deionized water was added to quench the reaction. After the mixture was extracted three times with ethyl acetate, the organic layer was washed with anhydrous sodium sulfate Dry for 8 hours, rotary evaporate the organic solvent to obtain a brown-red product, further purify the product by column chromatography using n-hexane:ethyl acetate (10:3), and then rotate the organic solution to obtain 1-(3-bromo Propyl)-indoline-2,3-dione (bromopropyl isatin);
[0072] (2) Synthesis of poly-p-terphenyl-bromopropyl isatin
[0073] Weigh 2 g of p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com