Sodium fluoride impregnated and coated vanadium-doped porous structure sodium ferric pyrophosphate positive electrode material and preparation method thereof
A technology of sodium iron pyrophosphate and positive electrode materials, which is applied in the field of lithium-ion battery technology and electrochemistry, can solve the problems of low specific capacity, achieve the effects of improving electrical conductivity, avoiding structural collapse, and excellent cycle performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, prepare Na 2 FeP 2 o 7 Material
[0032] First, take 0.9085g (5mmol) of ferrous oxalate dihydrate and 1.2121g (10mmol) of anhydrous sodium dihydrogen phosphate, put them into a polytetrachlorethylene ball mill jar, add acetone and ball mill for 6 hours, and then place them in a vacuum drying oven at 50°C After drying for 2 hours and grinding for 1 hour, the precursor powder was obtained.
[0033] The precursor powder is heated to 560°C at a heating rate of 2°C / min in a tube furnace with argon gas for 12 hours, and then cooled with the furnace. After the obtained product is ground, Na 2 FeP 2 o 7 Material.
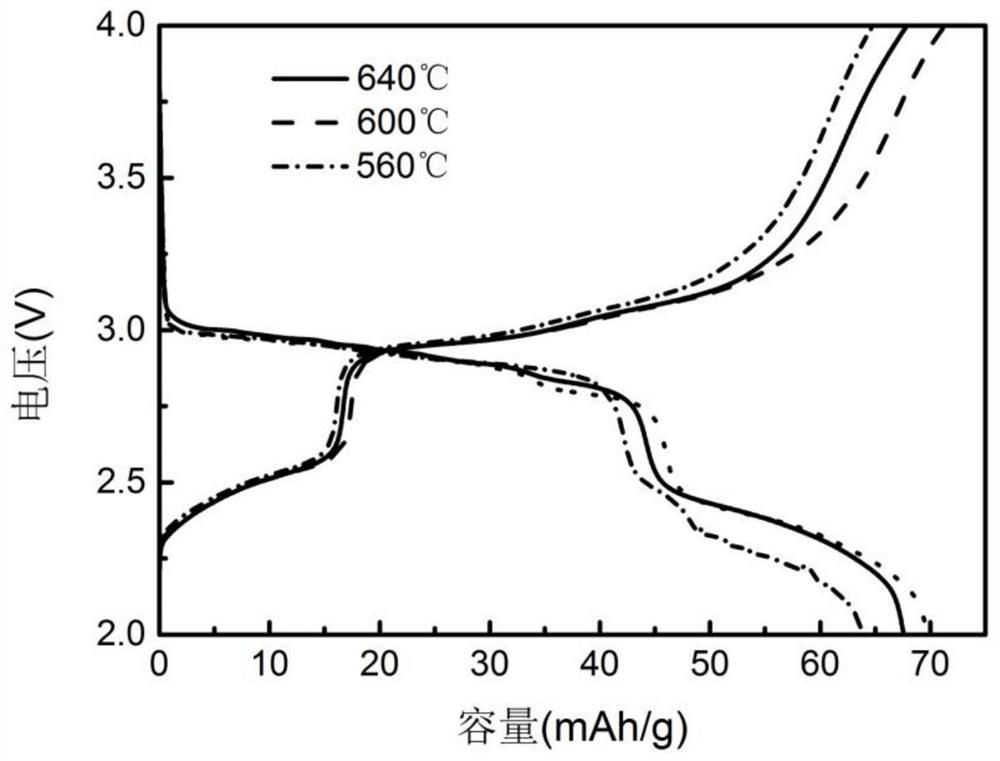
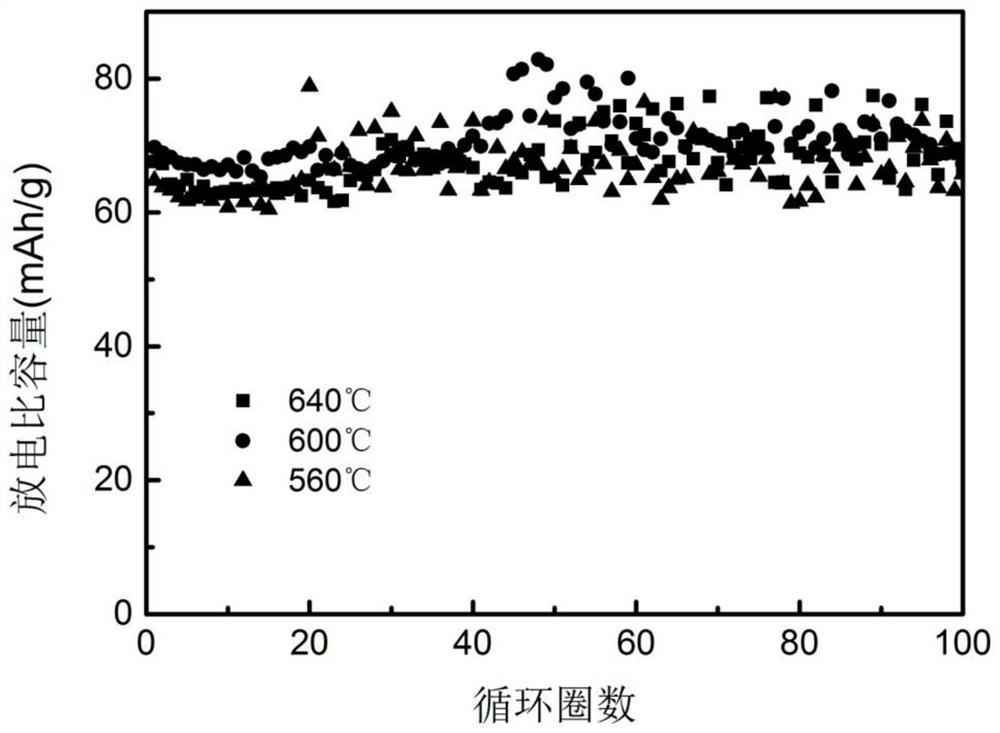
[0034] Adjust the sintering temperature in the above preparation method to 600°C and 640°C respectively, and prepare Na 2 FeP 2 o 7 Material. figure 1 Na obtained under the condition of 600℃ 2 FeP 2 o 7 The SEM image of the material, it can be seen from the image that the sodium iron pyrophosphate positive electrode material shows agglomera...
Embodiment 2
[0037] Embodiment 2, prepare Na 2 Fe 1-1.5x V x P 2 o 7 Material
[0038] First take 0.7722g (4.25mmol) of ferrous oxalate dihydrate, 1.2121g (10mmol) of anhydrous sodium dihydrogen phosphate and 0.0591g (0.5mmol) of ammonium metavanadate, put them into a polytetrachlorethylene ball mill jar, add acetone After ball milling for 6 hours, drying in a vacuum oven at 50°C for 2 hours, and grinding for 1 hour to obtain the precursor powder.
[0039] The precursor powder was heated to 600°C at a heating rate of 2°C / min in a tube furnace with argon gas for 12 hours, and then cooled with the furnace. The obtained product was ground to obtain Na 2 Fe 0.85 V 0.1 P 2 o 7 Material.
[0040] The amount of raw materials in the above preparation method is adjusted to 0.7041g (3.875mmol) ferrous oxalate dihydrate, 1.2121g (10mmol) anhydrous sodium dihydrogen phosphate, 0.0886g (0.75mmol) ammonium metavanadate, according to the same method as above Preparation of vanadium-doped sodiu...
Embodiment 3
[0044] Embodiment 3, preparation NaF@Na 2 Fe 0.775 V 0.15 P 2 o 7
[0045] First take 0.7041g (3.875mmol) of ferrous oxalate dihydrate, 1.2121g (10mmol) of anhydrous sodium dihydrogen phosphate and 0.0886g (0.75mmol) of ammonium metavanadate, put them into a polytetrachloroethylene ball mill tank, add acetone After ball milling for 6 hours, drying in a vacuum oven at 50°C for 2 hours, and grinding for 1 hour to obtain the precursor powder.
[0046] The precursor powder was heated to 600°C at a heating rate of 2°C / min in a tube furnace with argon gas for 12 hours, and then cooled with the furnace. The obtained product was ground to obtain Na 2 Fe 0.775 V 0.15 P 2 o 7 Material.
[0047] Na 2 Fe 0.775 V 0.15 P 2 o 7 The material and 0.0294g (0.49mmol) sodium chloride were added to deionized water, stirred evenly by ultrasonic, and then 49mL 0.01mol / L potassium fluoride aqueous solution was added dropwise through a peristaltic pump to obtain a suspension; the suspen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| retention rate | aaaaa | aaaaa |
| retention rate | aaaaa | aaaaa |
| retention rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com