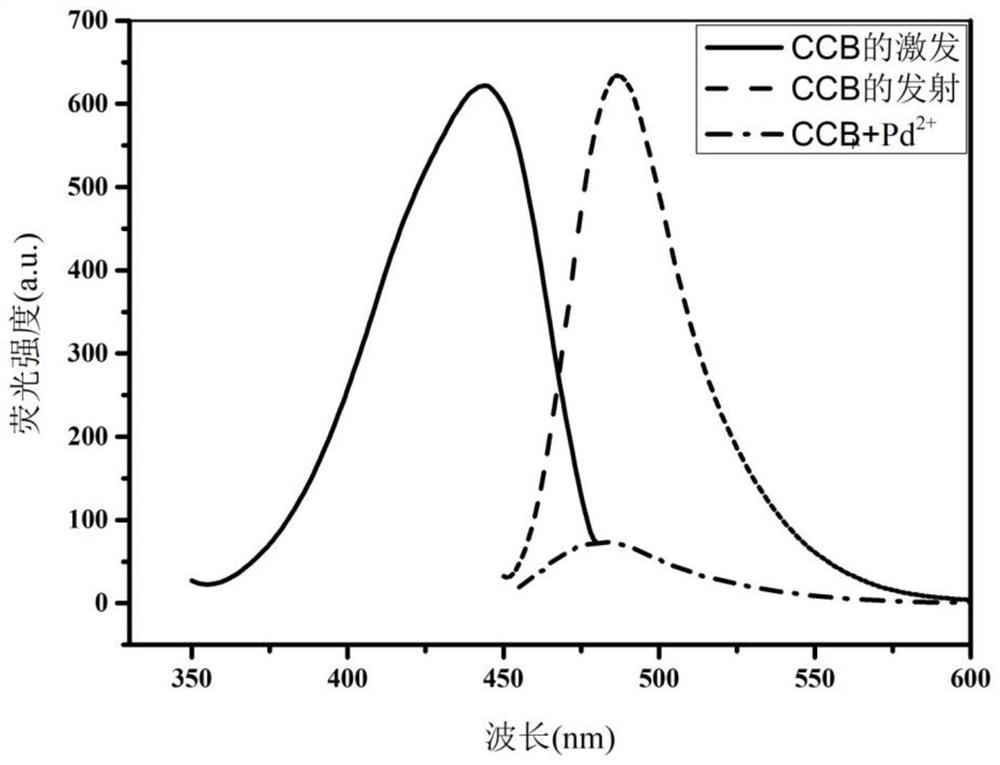
Coumarin-based palladium ion fluorescent probe compound and preparation method thereof
A technology for fluorescent probes and compounds, applied in the field of coumarin-based palladium ion fluorescent probe compounds and their synthesis, can solve many problems, and achieve the effects of simple post-processing, high sensitivity, and efficient synthesis methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of compound 3
[0036] To a solution of 4-(diethylamino)salicylaldehyde (0.193 g, 1 mmol) in ethanol (5 mL) was added diethyl malonate (0.52 mL, 3.4 mmol). Add 50 μL and two drops of glacial acetic acid in 4 mL ethanol to the reaction. The reaction was stirred at 80° C. for 24 h, water (30 mL) was added to the system, and extracted with ethyl acetate (3×20 mL). The combined organic phases were washed successively with water and saturated brine, and dried over anhydrous sodium sulfate. After the solvent was spin-dried, the obtained crude product was subjected to silica gel chromatography with petroleum ether / ethyl acetate as the eluent to obtain compound 3 as a yellow solid (0.323 g, 78%).
[0037] 1H NMR (400MHz, DMSO-d6) δ8.55(s, 1H), 7.63(d, J=9.0Hz, 1H), 6.77(dd, J=9.0, 2.4Hz, 1H), 6.53(d, J= 2.4Hz, 1H), 4.23(q, J=7.1Hz, 2H), 3.48(q, J=7.0Hz, 4H), 1.28(t, J=7.1Hz, 3H), 1.14(t, J=7.0Hz ,6H).13C NMR(101MHz,DMSO)δ163.85,158.51,157.50,15...
Embodiment 2
[0038] Embodiment 2: the preparation of compound 5
[0039] Compound 3 (0.29 g, 1 mmol) obtained in Example 1 was dissolved in 15 mL of methanol, hydrazine hydrate (240 μL, 4 mmol, 80%) was added to the system and stirred at room temperature for 12 min, the system was frozen in a refrigerator, and suction filtered to obtain yellow solid. The crude product was dried under vacuum for 12h to obtain compound 5 (0.22g, 80%).
[0040]1H NMR (400MHz, Chloroform-d) δ9.72(t, J=4.3Hz, 1H), 8.68(s, 1H), 7.43(d, J=8.9Hz, 1H), 7.27(s, 0H), 6.65 (dd, J=8.9,2.5Hz,1H),6.49(d,J=2.4Hz,1H),4.13(d,J=4.3Hz,2H),3.46(q,J=7.1Hz,4H),1.24 (t,J=7.1Hz,7H).13C NMR(101MHz,CDCl3)δ163.90,162.13,157.65,152.69,148.05,131.16,110.01,109.12,108.25,96.62,77.35,77.03,76.72,45.122,12.
Embodiment 3
[0041] Embodiment 3: the preparation of palladium ion fluorescent probe compound CCB
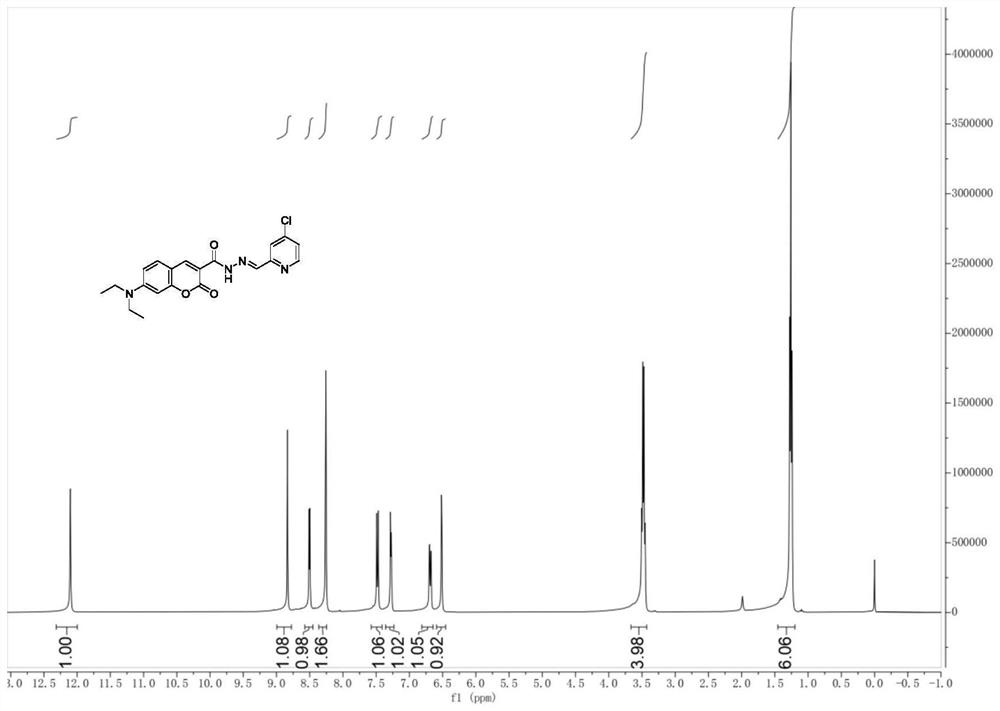
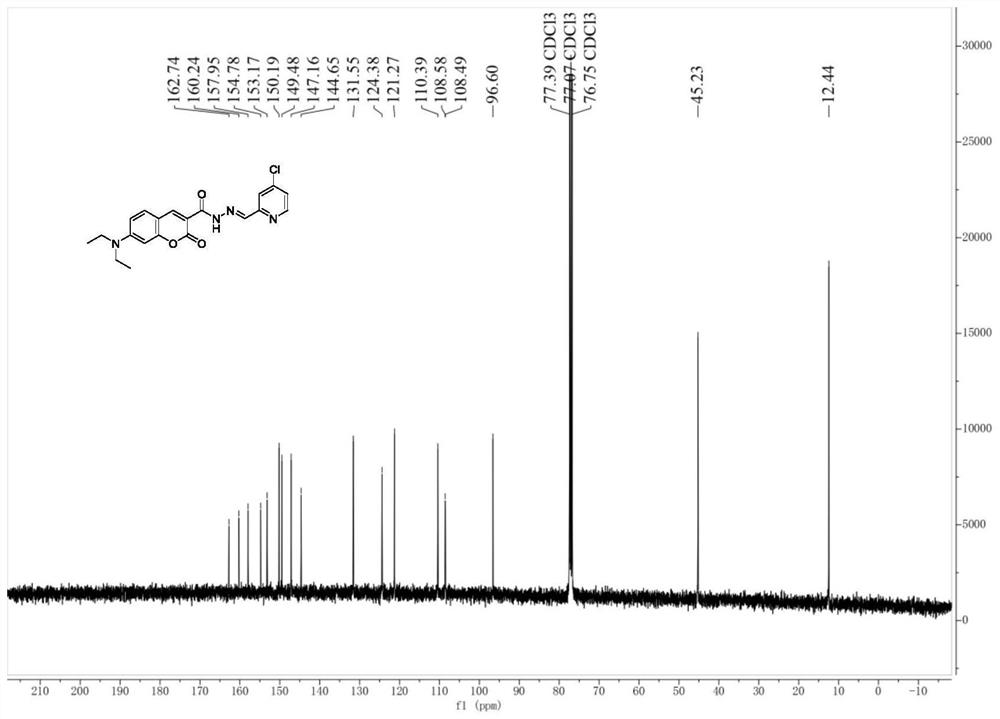
[0042] Under nitrogen protection, compound 5 (55 mg, 0.2 mmol) obtained in Example 2 was dissolved in 20 mL of methanol, 4-chloro-2-pyridinecarbaldehyde (28 mg, 0.2 mmol) was added, and heated to reflux for 12 h. The system was frozen in the refrigerator, and a yellow solid was obtained by suction filtration. The crude product was dried under vacuum for 12 hours to obtain the palladium ion fluorescent probe compound CCB (64mg, 80%). product of 1 H NMR spectrum and 13 The C NMR spectra are as follows figure 1 and as shown in Fig.
[0043] 1H NMR (400MHz, Chloroform-d) δ12.10(s,1H),8.84(s,1H),8.50(d,J=5.4Hz,1H),8.26(d,J=2.9Hz,2H),7.48 (d,J=8.9Hz,1H),7.28(dd,J=5.3,1.9Hz,1H),6.69(dd,J=9.0,2.5Hz,1H),6.52(d,J=2.5Hz,1H) ,3.48(q,J=7.1Hz,4H),1.26(t,J=7.1Hz,6H).13C NMR(101MHz,Chloroform-d)δ162.74,160.24,157.95,154.78,153.17,150.19,149.48,147.16, 144.65, 131.55, 124.38, 121.26, 110.39, 108.58, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com