Preparation method of amorphous tantalum oxide nanospheres
A tantalum oxide and amorphous technology, applied in the field of nanomaterials, can solve the problems of low photocatalytic efficiency, achieve excellent photocatalytic activity, high feasibility, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
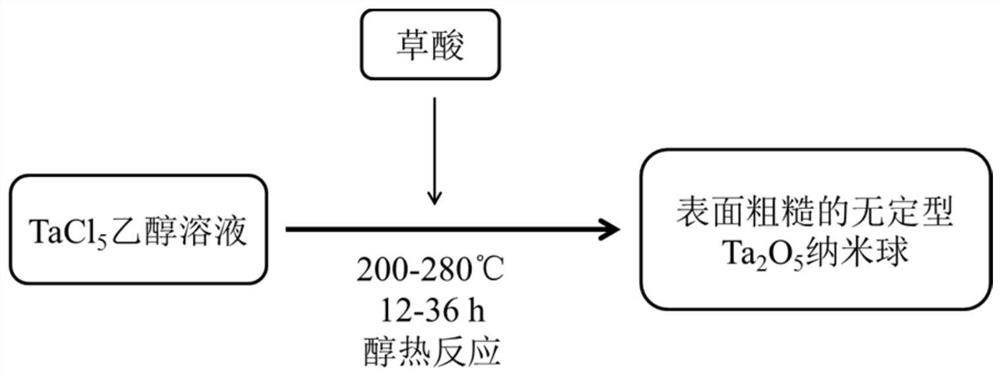
[0044] Such as figure 1 As shown, Example 1 proposes a rough surface amorphous Ta 2 o 5 The preparation method of nanosphere specifically comprises the following steps:
[0045] 1. Weigh 0.05mmol of TaCl 5 The raw materials were dissolved in 15mL of ethanol and mixed well.
[0046] 2. Add oxalic acid to the ethanol solution of tantalum chloride, the ratio of tantalum chloride to oxalic acid is 1:5, stir well to make the solution evenly mixed.
[0047] 3. Transfer the mixed solution into the autoclave for alcohol thermal reaction, the hydrothermal temperature is 240°C, and the hydrothermal time is 12h.
[0048] 4. The precipitate obtained from the alcohol thermal reaction was centrifuged at 4000rpm for 8min, and dried at 60°C for 4h. A smooth amorphous Ta 2 o 5 nanospheres.
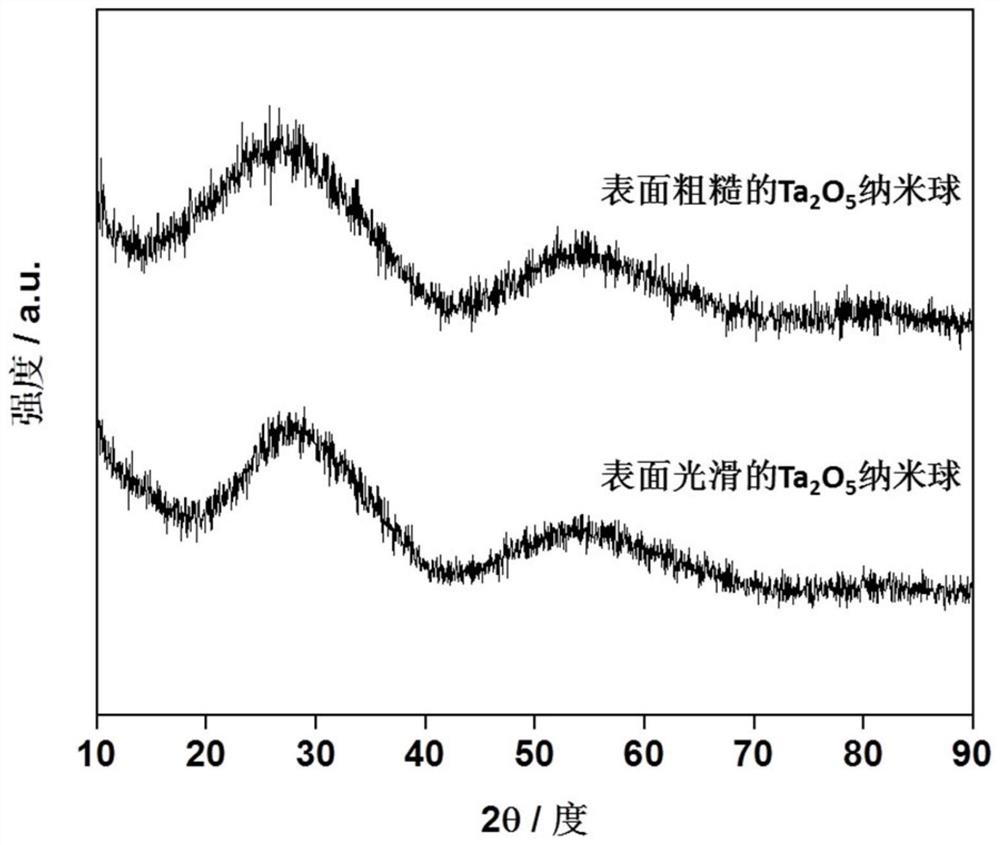
[0049] figure 2 The upper part of the XRD spectrum is the XRD spectrum of the product prepared in Example 1. It can be seen from the figure that the product has no obvious XRD characteristic peak...
Embodiment 2
[0052] Embodiment 2 proposes a smooth amorphous Ta 2 o 5 The preparation method of nanosphere specifically comprises the following steps:
[0053] 1. Weigh 0.05mmol of TaCl 5 The raw materials were dissolved in 15mL of ethanol and mixed well.
[0054] 2. Transfer the ethanol solution of tantalum chloride into the autoclave for alcohol thermal reaction, the hydrothermal temperature is 240°C, and the hydrothermal time is 12h.
[0055] 3. The precipitate obtained from the alcohol thermal reaction was centrifuged at 4000rpm for 8min, and dried at 60°C for 4h. A smooth amorphous Ta 2 o 5 nanospheres.
[0056] figure 2 The lower part of the XRD pattern is the XRD pattern of the product prepared in Example 2. It can be seen from the figure that the alcohol thermal product in Example 2 is also in an amorphous state. Figure 4 Bottom infrared spectrum is the infrared spectrum of the product that embodiment 2 makes, as can be seen from the figure compared with the infrared spec...
Embodiment 3
[0058] This embodiment provides a rough surface amorphous Ta 2 o 5 The preparation method of nanospheres is to adjust the amount of oxalic acid added in the system, and to explore the influence of the amount of oxalic acid on the shape of the product. The specific steps are as follows.
[0059] 1. Weigh 0.05mmol of TaCl 5 The raw materials were dissolved in 15mL of ethanol and mixed well.
[0060] 2. Add oxalic acid to the ethanol solution of tantalum chloride, the ratio of tantalum chloride to oxalic acid is 1:1, 1:10, stir well to make the solution evenly mixed.
[0061] 3. Transfer the mixed solution into the autoclave for alcohol thermal reaction, the hydrothermal temperature is 240°C, and the hydrothermal time is 12h.
[0062] 4. The precipitate obtained from the alcohol thermal reaction was centrifuged at 4000rpm for 8min, and dried at 60°C for 4h.
[0063] The obtained product is amorphous Ta with rough surface 2 o 5 nanospheres and the particle diameter obtained ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com