Metformin hydrochloride dual sustained and controlled release composition as well as preparation method and application thereof
A technology of metformin hydrochloride and its composition, which is applied in the field of pharmaceutical preparations, can solve the problems of high production cost, reduced stability, and serious slow-release tablets, and achieve the effects of low production cost, improved compliance, and easy swallowing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1. A kind of preparation method of film-controlled metformin double controlled-release tablet
[0076] 1. Weigh the components according to the formula of the tablet core and the formula of the coating solution
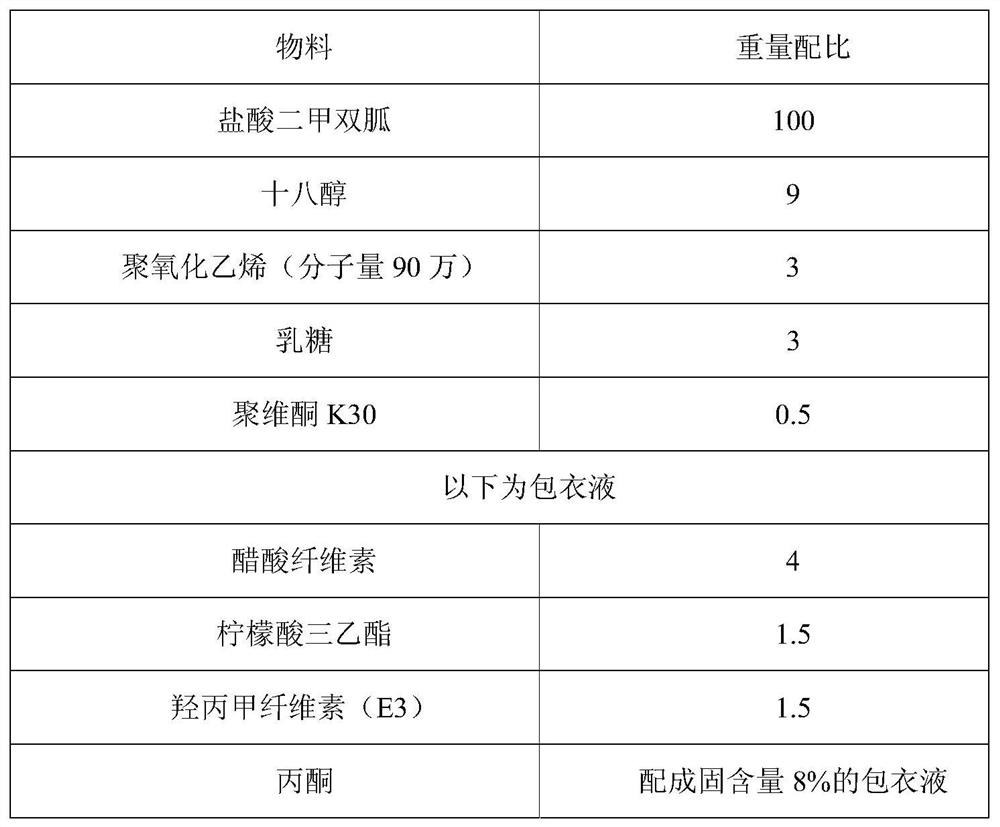
[0077] Table 1 The weight ratio table of the components of the film-controlled metformin dual controlled-release tablet
[0078]
[0079] 2. Preparation steps
[0080] 1. Preparation of sustained-release metformin hydrochloride tablet cores
[0081] (1) Metformin hydrochloride raw material and lactose, stearyl alcohol, polyethylene oxide are pulverized through a 100 mesh sieve;
[0082] (2) Put the powder sieve obtained in (1) into a wet granulator, stir evenly at a low speed, add 5% povidone K30 aqueous solution to make a soft material, and make it in a 12-mesh oscillating granulator with a frequency of 40-50Hz. grain;
[0083] (3) Dry the wet granules at 60°C, control the water content to 2.5% to 3.5%, take out, and granulate in a swinging g...
Embodiment 2
[0087] Embodiment 2. A kind of preparation method of film-controlled metformin double controlled-release tablet
[0088] 1. Weigh the components according to the formula of the tablet core and the formula of the coating solution
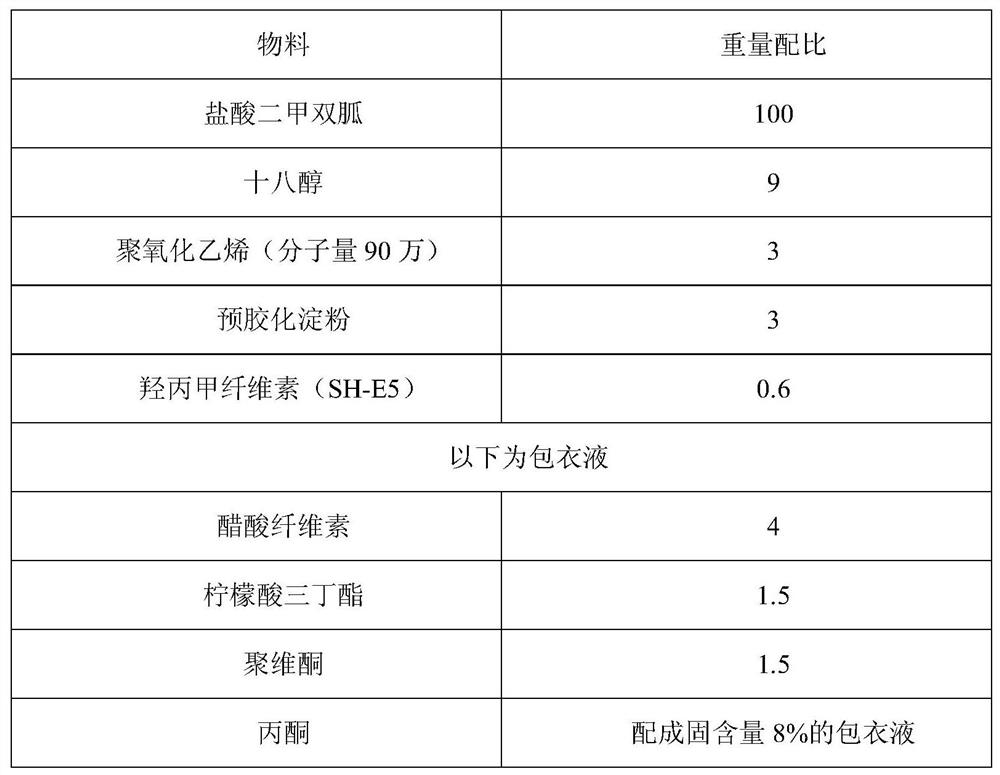
[0089] Table 2 The weight ratio of the components of the film-controlled metformin dual controlled-release tablet
[0090]
[0091] 2. Preparation steps
[0092] 1. Preparation of sustained-release metformin hydrochloride tablet cores
[0093] (1) Metformin hydrochloride raw material and lactose, stearyl alcohol, polyethylene oxide are pulverized through 80 mesh sieves;
[0094] (2) Put the powder sieve obtained in (1) into a wet granulator, stir evenly at a low speed, add 3% hypromellose aqueous solution to make a soft material, and use a 12-mesh oscillating granulator with a frequency of 40-50Hz Granulation;
[0095](3) Dry the wet granules at 60°C, control the water content to 2.5% to 3.5%, take out, and granulate in a swinging granulator w...
Embodiment 3
[0099] Embodiment 3 A kind of preparation method of film-controlled metformin double controlled-release tablet
[0100] 1. Weigh the components according to the formula of the tablet core and the formula of the coating solution
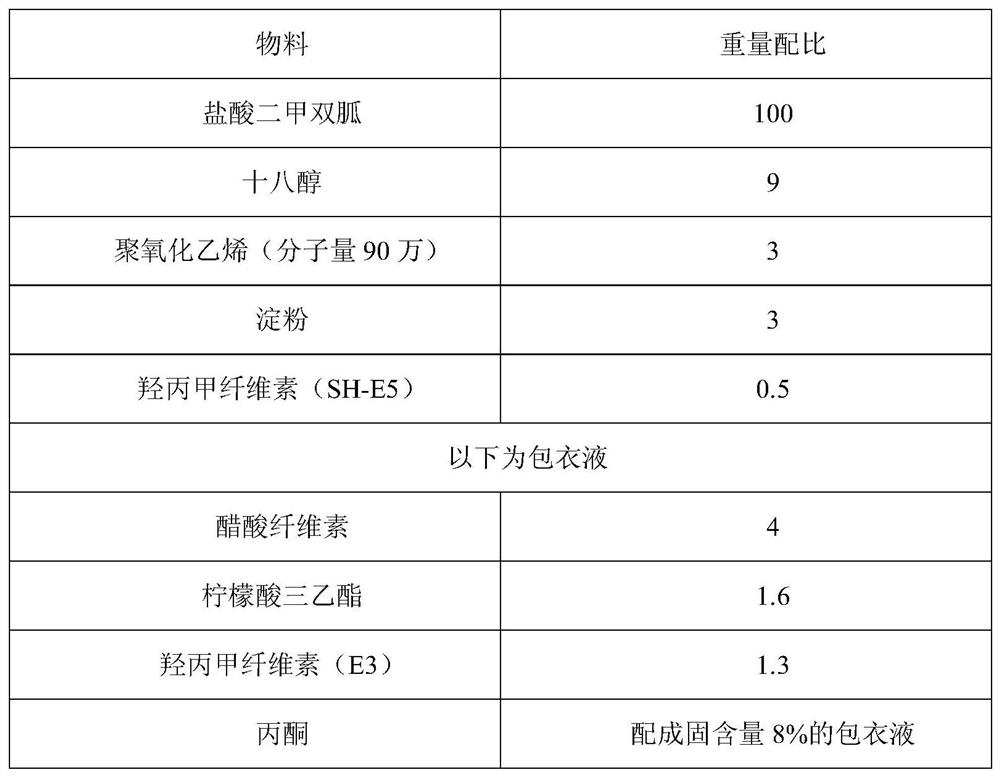
[0101] Table 3 The weight ratio table of the components of the film-controlled metformin dual controlled-release tablet
[0102]
[0103] 2. Preparation steps
[0104] 1. Preparation of sustained-release metformin hydrochloride tablet cores
[0105] (1) Metformin hydrochloride raw material and lactose, stearyl alcohol, polyethylene oxide are pulverized through a 120 mesh sieve;
[0106] (2) Add the powder sieve obtained in (1) into a wet granulator, stir evenly at a low speed, add 3% hydroxypropyl methylcellulose aqueous solution, mix the powder sieve and add a binder to make a soft material, Granulate in a 12-mesh oscillating granulator with a frequency of 40-50Hz;
[0107] (3) Dry the wet granules at 60°C, control the water content to 1.5% to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com