Synthesis method of diphenylamine L57 and L67
A synthetic method, the technology of diphenylamine, applied in the field of medicinal chemistry, can solve the problems of unsatisfactory product quality, yield and production cost, low catalytic activity of activated clay, no decolorization of catalyst, etc., and achieve outstanding economy and environmental protection , high economy and environmental protection, easy to separate the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
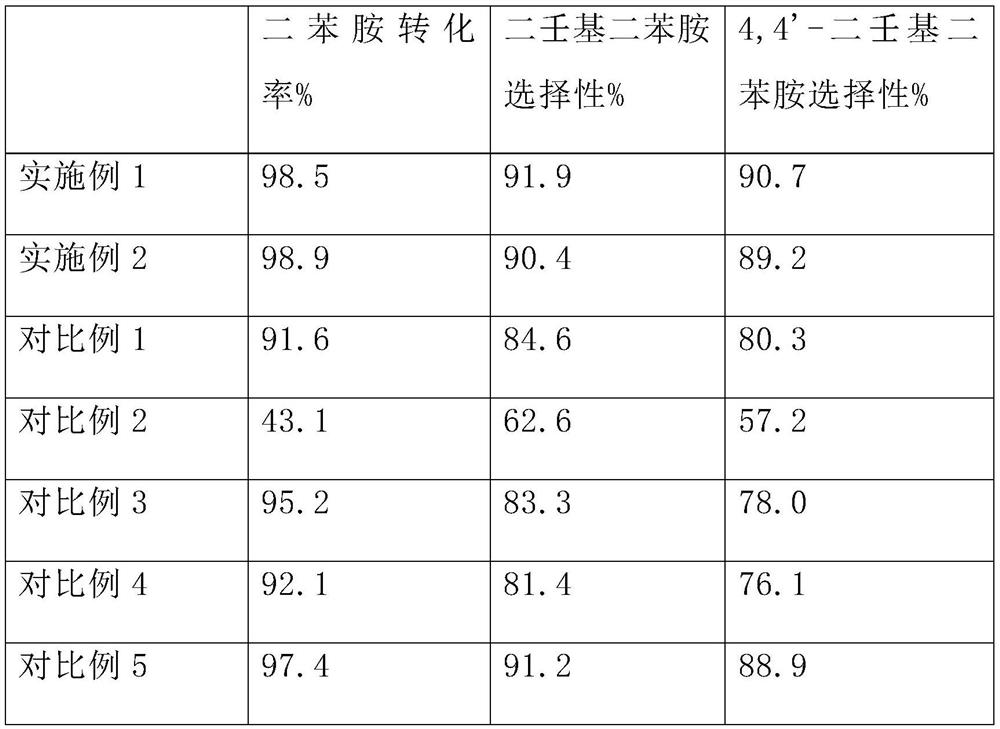
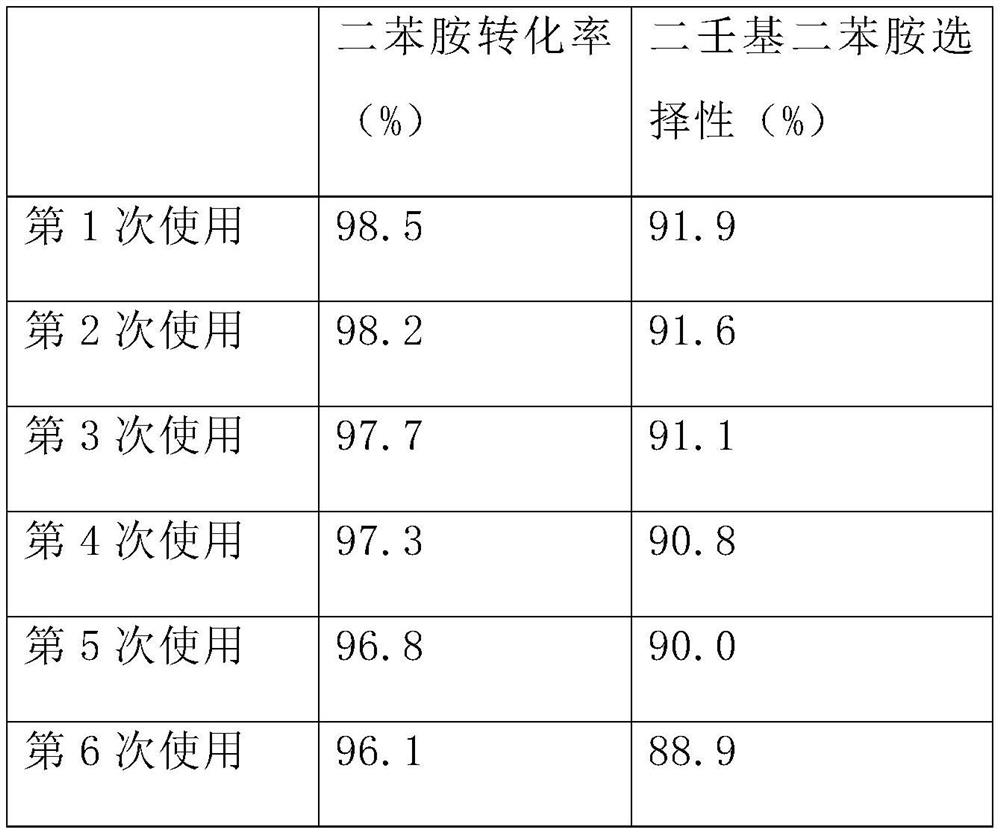
Examples
preparation example 1
[0028] Preparation Example 1: AlCl 3 -SnCl 2 Preparation of Two-Component Supported Catalyst
[0029] The sepiolite was calcined at 700°C for 6h in a muffle furnace to activate it. Soak the activated sepiolite in 10% sulfuric acid solution for 24 hours, then centrifuge to separate solid particles, wash thoroughly with water and absolute ethanol in sequence, and dry in vacuum for 4 hours to obtain a catalyst carrier. Take 20 g of the catalyst carrier, add 250 mL of carbon tetrachloride, then add 6.0 g of anhydrous aluminum trichloride and 2.0 g of anhydrous stannous chloride, and stir for 2 h under reflux. Filter, wash with carbon tetrachloride 3 times, and vacuum-dry at 75°C for 10h, then place in a muffle furnace and calcinate at 280°C for 3h to obtain AlCl 3 -SnCl 2 Two-component supported catalyst. The loading was measured by atomic absorption spectroscopy to be 25.6% (calculated based on sepiolite), and the mass ratio of aluminum trichloride to stannous chloride was 1...
preparation example 2
[0030] Preparation Example 2: AlCl 3 -SnCl 2 Preparation of Two-Component Supported Catalyst
[0031] The sepiolite was calcined at 700°C for 6h in a muffle furnace to activate it. Soak the activated sepiolite in 15% sulfuric acid solution for 24 hours, then centrifuge to separate solid particles, wash thoroughly with water and absolute ethanol in sequence, and dry in vacuum for 4 hours to obtain a catalyst carrier. Take 20g of the catalyst carrier, add 250mL of carbon tetrachloride, then add 7.5g of anhydrous aluminum trichloride and 2.5g of anhydrous stannous chloride into it, and stir for 2h under reflux. Filter, wash with carbon tetrachloride 3 times, and vacuum-dry at 75°C for 10h, then place in a muffle furnace and calcinate at 280°C for 3h to obtain AlCl 3 -SnCl 2 Two-component supported catalyst. Using atomic absorption spectrometry, the loading capacity was measured to be 29.4% (calculated based on sepiolite), and the mass ratio of aluminum trichloride to stannou...
Embodiment 1
[0036] Embodiment 1: the synthesis of nonyl diphenylamine (antioxidant L67)
[0037] Add 169.0 grams of diphenylamine, 252.5 grams of nonene in the autoclave, the catalyst 15g of Preparation Example 1, the hydrogenated cardanol-based polymerization inhibitor 5-pentadecyl-2-(((4-(phenylamino )phenyl)amino)methyl)phenol, replace the gas in the system with nitrogen several times, close the reactor, heat to 80°C to melt the diphenylamine and start stirring, then further heat up to 170°C for reaction, the reaction pressure is controlled At 0.4MPa. As the reaction proceeds, the pressure in the reactor decreases gradually. When the reaction pressure drops to 0.2MPa, 126.2g nonene is added to continue the reaction for 2 hours, and then 126.2g nonene is added again to continue the reaction for 4 hours. Stop reaction afterwards, reclaim described AlCl with fiber membrane filtration 3 -SnCl 2 Two-component supported catalyst, liquid phase sampling and analysis of components, and vacuu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com