Bifidobacterium breve capable of relieving diarrhea and application thereof
A technology of Bifidobacterium breve and diarrhea, applied in the field of microorganisms, to achieve the effect of reducing the level of pro-inflammatory factors, increasing the diversity of intestinal flora, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Screening and strain identification of Bifidobacterium breve CCFM1151
[0056] 1. Screening
[0057] Taking healthy human feces samples from Fengqiu area, Henan Province, take 0.5g samples preserved in 30% (v / v) glycerol, add 4.5mL normal saline (containing 0.5g / L cysteine acid) in a 10mL centrifuge tube to obtain 10 -1 Diluent, repeat the above dilution steps to obtain 10 -2 、10 -3 、10 -4 、10 -5 、10 -6 Dilution: Pipette 100 μL of gradient dilutions of different gradients and spread them on MRS solid medium (containing 0.5g / L cysteine), incubate at 37°C for 72 hours to obtain a dilution-coating plate; pick a dilution-coating plate The typical colonies above were streaked on the MRS solid medium (containing 0.5 g / L cysteine), cultured at 37°C for 48 hours, and purified colonies were obtained; the purified colonies were picked and inoculated into MRS liquid medium (containing 0.5 g / L cysteine), cultured at 37°C for 48 hours to obtain strain CCFM1151.
...
Embodiment 2~5
[0060] In embodiment 2~5, the preparation method of lactic acid bacteria liquid is as follows:
[0061] Dip the lactic acid bacteria liquid to streak on the MRS solid medium, culture at 37°C for 48 hours to obtain a single colony; pick a single colony and insert it into the MRS liquid medium, and cultivate it at 37°C for 24 hours to obtain an activation solution; Inoculate the inoculum of % (v / v) into the MRS liquid medium, and cultivate it at 37°C for 24 hours to obtain the primary seed liquid; insert the primary seed liquid into the MRS liquid culture according to the inoculation amount of 1% (v / v) culture medium at 37°C for 24 hours to obtain a secondary seed liquid; insert the secondary seed liquid into the MRS liquid medium according to the inoculation amount of 1% (v / v), and cultivate at 37°C for 24 hours to obtain a bacterial liquid; The bacterial solution was centrifuged at 6000g for 15min to collect the precipitate; the precipitate was washed twice with PBS buffer sol...
Embodiment 2
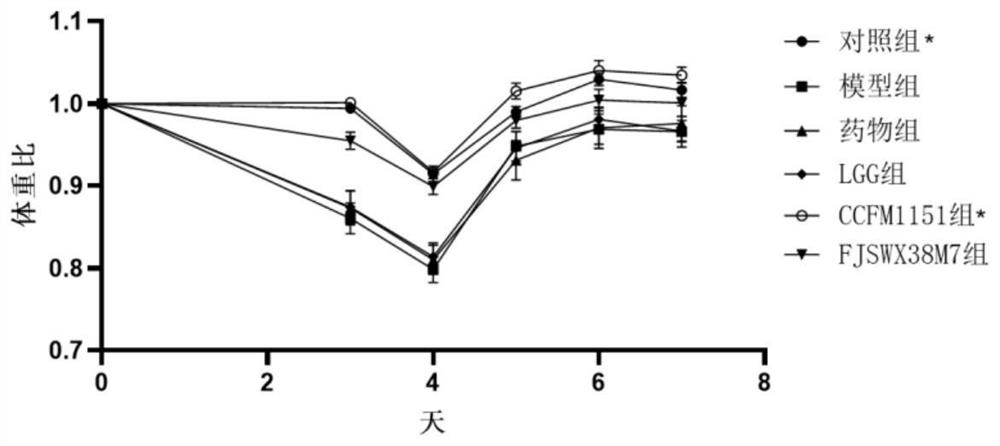
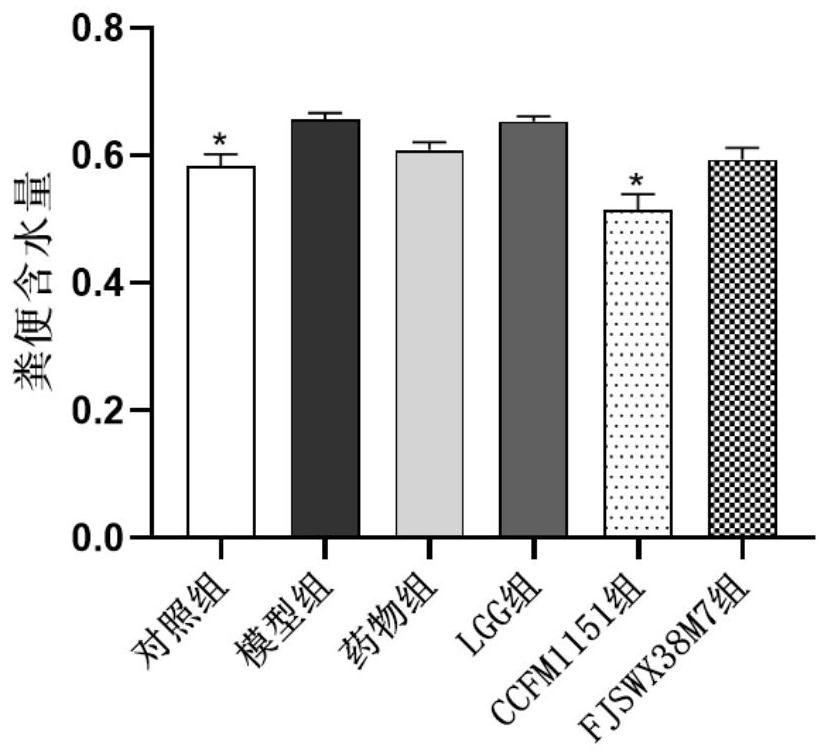
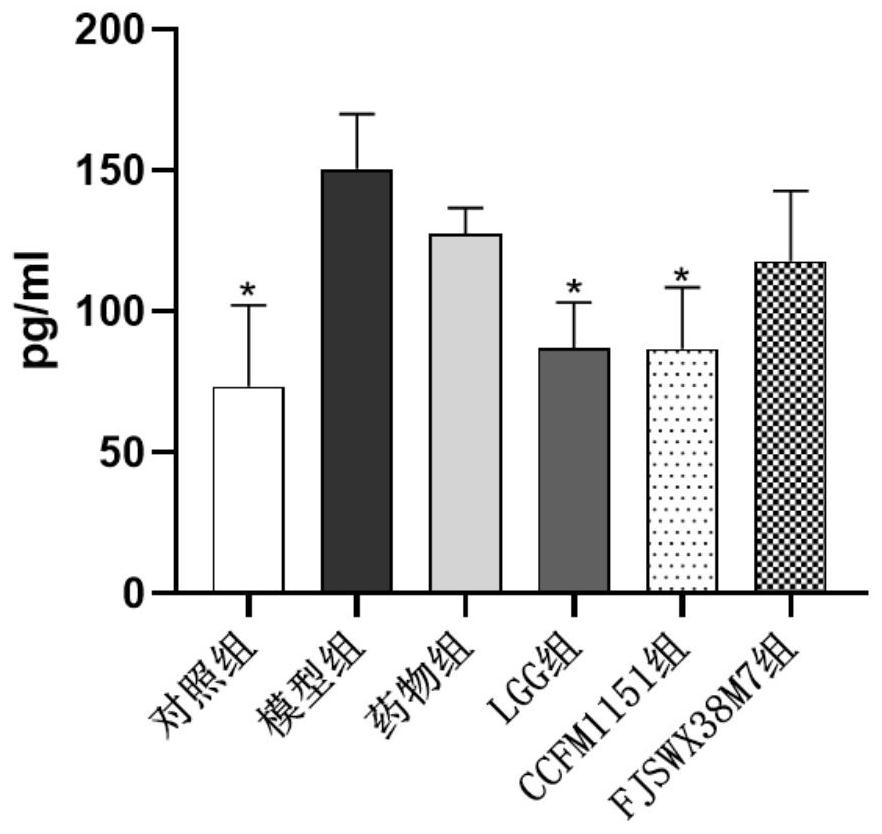
[0063] Example 2: Effects of Bifidobacterium breve CCFM1151 on body weight and feces water content of mice with diarrhea
[0064] Specific steps are as follows:
[0065] Take 48 female pathogen-free (SPF) BALB / c mice aged 3-4 weeks, and raise them under the conditions of room temperature 22-24°C, humidity 40-60%, 12h / 12h day and night, free access to food and water After feeding for 1 week, they were randomly divided into 6 groups with 8 rats in each group. The 6 groups were respectively: control group, model group, ciprofloxacin-administered drug group, Lactobacillus rhamnosus (Lactobacillus rhamnosus) GG LGG group, the CCFM1151 group of oral administration of Bifidobacterium breve (Bifidobacterium breve) CCFM1151 bacterium liquid, the FJSWX38M7 group of oral administration of Bifidobacterium breve (Bifidobacterium breve) FJSWX38M7 bacterial liquid, wherein Bifidobacterium breve FJSWX38M7 is screened according to the same method as in Example 1 Methods The feces samples of i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com