Method for extracting nickel from laterite-nickel ore and mineralizing CO2 simultaneously
A laterite nickel ore, time technology, applied in nickel sulfate, process efficiency improvement, magnesium carbonate and other directions, can solve the problem of high energy consumption for mineralization, and achieve the effects of reducing environmental pollution, mild reaction conditions and saving production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
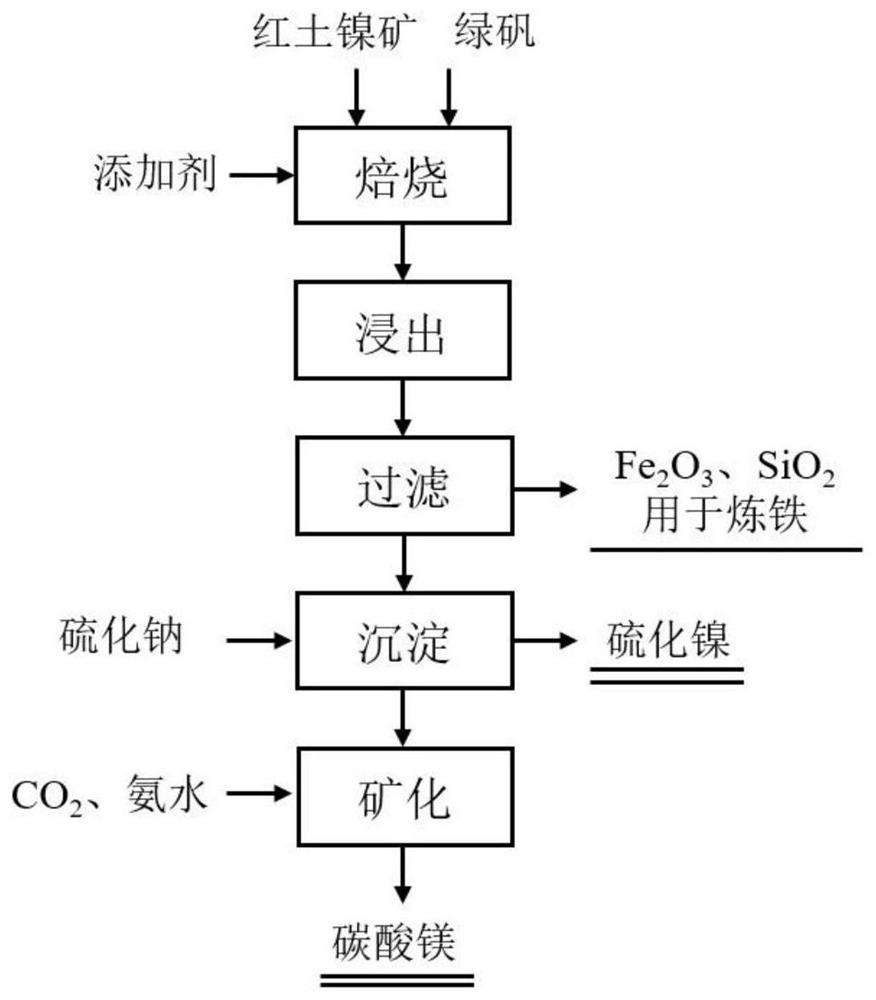
[0021] (1) Evenly mix the laterite nickel ore that is finely ground to below 150 μm with green vitriol and sodium sulfate, and control the mass ratio of laterite nickel ore and green vitriol to be 1:4; the content of sodium sulfate is 5% of the total material quality; the mixture Calcined at 650°C for 180min to obtain a solid product;
[0022] (2) The solid product obtained in step 1 is leached with water at 100° C., and the leached slurry is subjected to solid-liquid separation to obtain a filter residue (the main component is Fe 2 o 3 , SiO 2 ) and a leach solution containing magnesium sulfate and nickel sulfate;
[0023] (3) Send the leachate obtained in step 2 to a sedimentation tank and add sodium sulfide in an equimolar amount to nickel ions in the leachate at 20° C. for a precipitation reaction to obtain a nickel sulfide product and a precipitation mother liquor;
[0024] (4) Slowly add ammonia water to the precipitated mother liquor obtained in step 3, so that the p...
Embodiment 2
[0026] (1) Evenly mix the laterite nickel ore that is finely ground to below 150 μm with green vitriol, iron sulfide, and sulfur, and control the mass ratio of laterite nickel ore to green vitriol to be 1:1; the contents of iron sulfide and sulfur are respectively 1% of the total material mass % and 5%; the mixture was roasted at 500°C for 240min to obtain a solid product;
[0027] (2) The solid product obtained in step 1 is leached with water at 25° C., and the leached slurry is separated from solid and liquid to obtain a filter residue (the main component is Fe 2 o 3 , SiO 2 ) and a leach solution containing magnesium sulfate and nickel sulfate;
[0028] (3) Send the leachate obtained in step 2 to a sedimentation tank and add sodium sulfide with twice the molar amount of nickel ions in the leachate to carry out precipitation reaction at 60° C. to obtain a nickel sulfide product and a precipitation mother liquor;
[0029] (4) Slowly add ammonia water to the precipitated moth...
Embodiment 3
[0031] (1) Evenly mix the laterite nickel ore that is finely ground to below 150 μm with green vitriol and potassium sulfate, and control the mass ratio of laterite nickel ore and green vitriol to be 1:8; the content of potassium sulfate is 25% of the total material quality; mix the mixture Calcined at 900°C for 30 minutes to obtain a solid product;
[0032] (2) The solid product obtained in step 1 is leached with water at 60° C., and the leached slurry is subjected to solid-liquid separation to obtain a filter residue (the main component is Fe 2 o 3 , SiO 2 ) and a leach solution containing magnesium sulfate and nickel sulfate;
[0033] (3) Send the leachate obtained in step 2 to a sedimentation tank and add sodium sulfide with three times the molar amount of nickel ions in the leachate to carry out a precipitation reaction at 40° C. to obtain a nickel sulfide product and a precipitation mother liquor;
[0034] (4) Slowly add ammonia water to the precipitated mother liquor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com