Brush polymer modified graphene oxide-immobilized capture receptor compound and application thereof in enrichment of monoclonal antibodies
A polymer and graphene technology, applied in measurement devices, instruments, scientific instruments, etc., can solve the problems of small carrier specific surface area, limited application, large steric hindrance, etc., to save experimental costs, improve detection sensitivity, and reduce space. The effect of steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
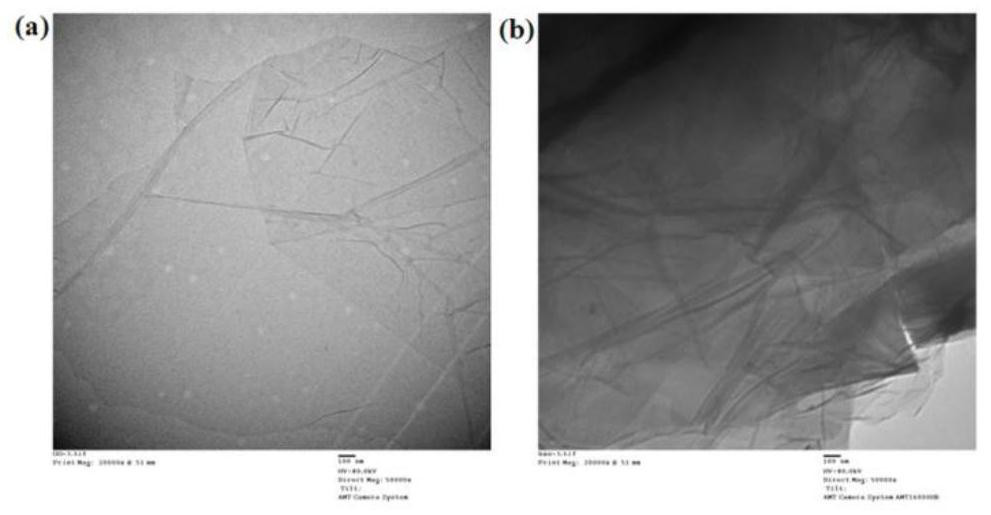
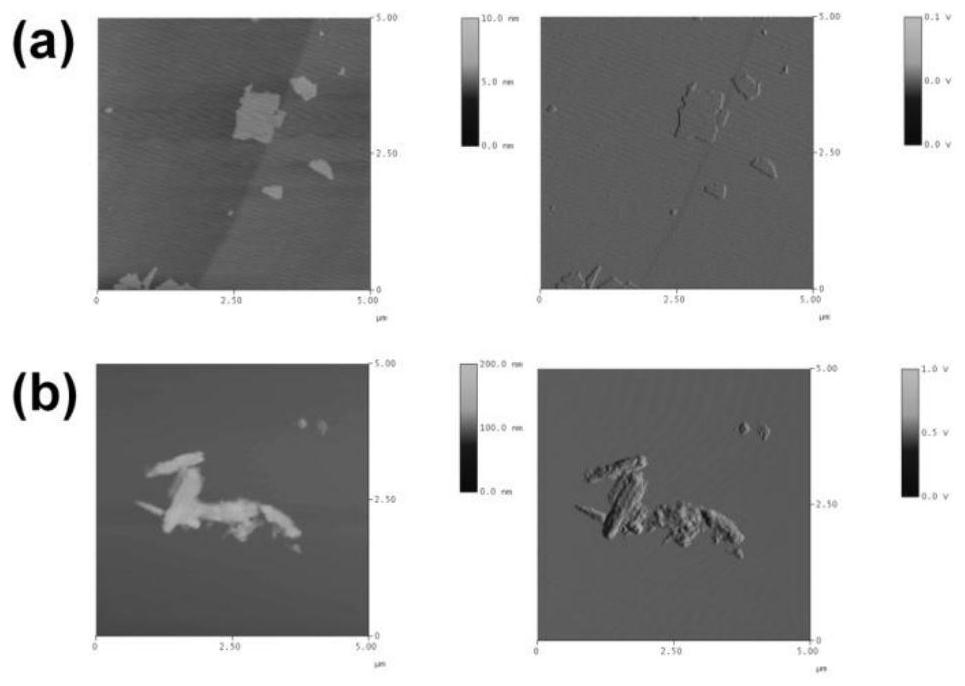
[0032] Embodiment 1, the preparation of brush polymer modified GO
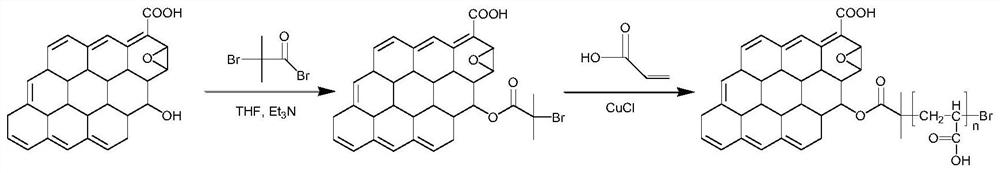
[0033] The reaction equation is as figure 1 shown.
[0034] Modification of ATRP initiator on GO surface: 100 mg of GO, 50 mL of freshly prepared tetrahydrofuran and 0.36 mL of triethylamine were placed in a 250 mL round bottom flask and vigorously stirred at 0-5 °C for 30 min under nitrogen atmosphere. Then 2-bromoisobutyryl bromide (0.32 mL, the mass ratio of graphene oxide to 2-bromoisobutyryl bromide is 1:0.006) was slowly added dropwise into the above reaction system. The reaction was stirred at this temperature for an additional 30 min before its temperature was raised to room temperature and stirring was continued for 4 h. The product was washed well with dichloromethane and dried in a vacuum oven at 50°C overnight.
[0035] Then SI-ATRP initiation was carried out on the surface of the above product: first, 50 mg of GO immobilized with the initiator was mixed with acrylic acid (2M), cupric chloride (0....
Embodiment 2
[0040] Example 2, GO-PAA immobilized capture receptor ErBb2
[0041] First, activate the terminal carboxyl group of GO-PAA: add 500 μL of NHS / EDC solution (400 mM EDC / 100 mM NHS), sonicate for 5 min, and mix at room temperature for 30 min. Subsequently, it was centrifuged at 10,000 rpm for 10 min to obtain the supernatant, and the material was washed twice with deionized water and PBS buffer respectively. Finally, 1 mL of trypsin solution (1 mg / mL) was added to the above GO-PAA polymer, and mixed overnight at 4°C. Calculated by BCA method, the loading capacity of GO-PAA on protein can reach 773.9mg / g.
Embodiment 3
[0042] Example 3, GO-PAA immobilized capture receptor ErBb2 (GO-ErBb2) enriched T-DM1
[0043] First determine the mass spectrometry detection conditions of T-DM1:
[0044] The theoretical values of the MRM transitions of T-DM1 and its internal standard were calculated using Skyline software, as shown in Table 1 and Table 2, respectively. Under the selected transition conditions, use ESI ion source, positive ion mode, and multi-stage reaction monitoring (MRM) to optimize the chromatographic conditions as shown in Table 3: Waters ACQUITY UPLC BEH C 18 (50mm×2.1mm, 1.7μm) chromatographic column; mobile phase: A: water (0.1% formic acid) B: acetonitrile (0.1% formic acid); flow rate 0.3mL / min; injection volume 10μL. The temperature of the injector was controlled at 4°C. Obtain the spectrogram of T-DM1 under optimized chromatographic mass spectrometry conditions, such as Figure 5 shown.
[0045] Table 1 MRM conditions of T-DM1
[0046]
[0047] Table 2 MRM conditions of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com