Improved preparation method of ketorolac intermediate
An intermediate, ketorolac technology, applied in the field of medicinal chemistry, can solve the problems of unsuitability for large-scale industrial production, difficult removal of high-boiling acetic acid solvent, difficult and cumbersome operation of liquid separation and extraction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
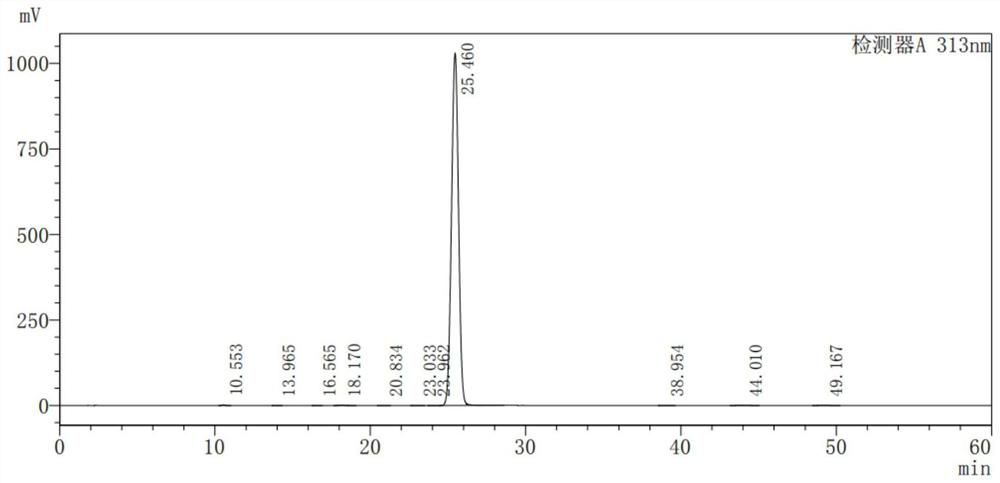
[0042] Add 25g of 2-benzoylpyrrole, 50.8g of triethyl methanetricarboxylate, 58.7g of manganese acetate dihydrate (trivalent), 24.0g of sodium acetate, and 111.8g of acetic anhydride into 250mL of toluene. , add about 125mL of sodium bisulfite aqueous solution (add 2.5g of sodium bisulfite to 125mL of water for dissolving configuration), separate the liquid, concentrate the organic phase under reduced pressure, directly use 50mL of isopropanol to make a slurry, filter, and dry to obtain 55.2 g Diethyl 5-benzoyl-2,3-dihydro-1H-pyrrolazine-1,1-dicarboxylate (intermediate), yield 94.2%. As detected by HPLC, the purity of the intermediate is 99.733%, and the single largest impurity is 0.091%. The results are shown in Table 1 and figure 1 .
[0043] Table 1 gained intermediate HPLC detection result
[0044] peak number keep time area high area% Resolution (USP) Number of Theoretical Plates (USP) 1 10.553 22554 1758 0.069 -- 15011 2 13.965 11...
Embodiment 2
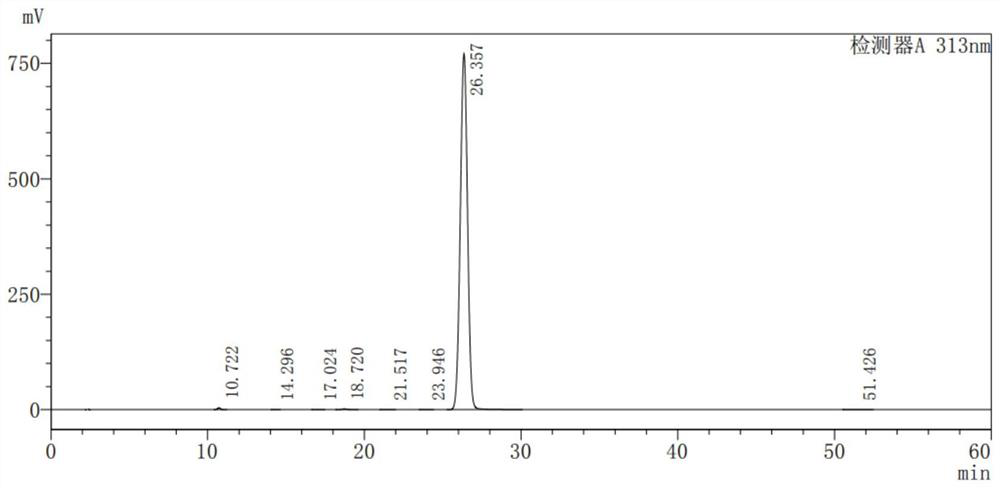
[0046] Add 56g of 2-benzoylpyrrole, 115.3g of triethyl methanetricarboxylate, 175.4g of manganese acetate dihydrate (trivalent), 53.8g of sodium acetate, and 267.5g of acetic anhydride into 504mL of N-methylpyrrolidone, After heating for complete reaction, add about 280 mL of sodium bisulfite aqueous solution (add 20.4 g of sodium bisulfite to 280 mL of water for dissolving configuration), separate liquids, and concentrate the organic phase under reduced pressure, directly use 280 mL of n-propanol to make a slurry, filter, After drying, 119.5 g of the intermediate 5-benzoyl-2,3-dihydro-1H-pyrrolazine-1,1-dicarboxylate diethyl ester was obtained, with a yield of 91.0%. As detected by HPLC, the purity of the intermediate is 99.570%, and the single largest impurity is 0.208%. The results are shown in Table 2 and figure 2 .
[0047] Table 2 gained intermediate HPLC detection result
[0048] peak number keep time area high area% Resolution (USP) Number of ...
Embodiment 3
[0050] Add 32g of 2-benzoylpyrrole, 49.3g of triethyl methanetricarboxylate, 90.2g of manganese acetate dihydrate (trivalent), 43.2g of sodium acetate, and 165.3g of acetic anhydride into 256mL of toluene. , add 160mL of sodium bisulfite aqueous solution (8.7g of sodium bisulfite is added to 160mL of water for dissolving configuration), liquid separation, after the organic phase is concentrated under reduced pressure, directly use 96mL of isopropanol to beat, filter, and dry to obtain 69.2g Diethyl 5-benzoyl-2,3-dihydro-1H-pyrrolazine-1,1-dicarboxylate, yield 92.3%. As detected by HPLC, the purity is 99.451%, and the largest single impurity is 0.186%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com