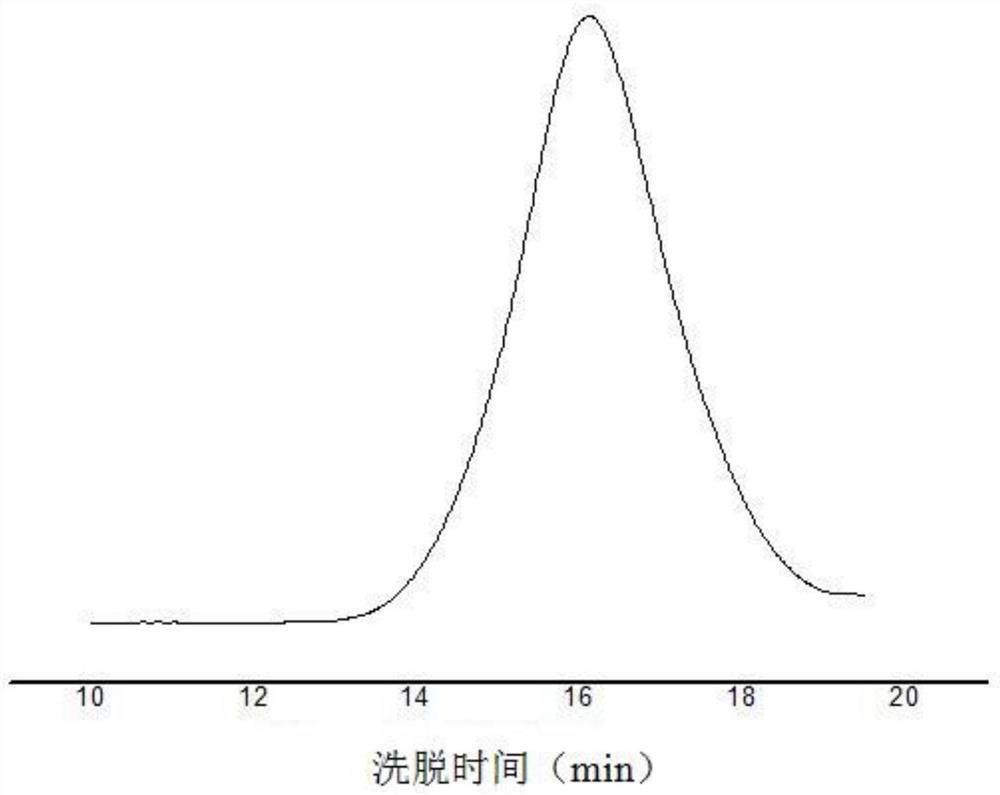
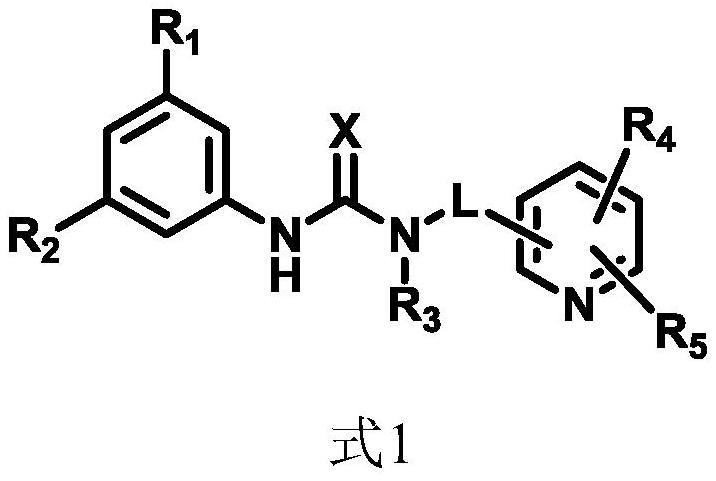
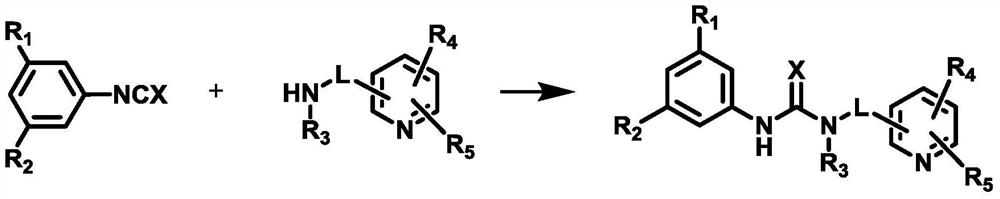
Organic bifunctional catalyst and preparation method thereof as well as stereoregular biodegradable polyester and preparation method thereof
A bifunctional catalyst, catalyst technology, applied in organic compound/hydride/coordination complex catalysts, organic chemistry, chemical instruments and methods, etc., can solve the problems of difficult physical and chemical properties, lack of functionalized side groups in polyester, etc. , to achieve the effect of optimized polymerization temperature, fast polymerization speed and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0101] The preparation method of acid anhydride in O-carboxyl ring:
[0102]
[0103] Dissolve α-hydroxy acid (10mmol) in 30mL of anhydrous tetrahydrofuran, add 200mg of activated carbon, 6.7mmol of triphosgene, stir at room temperature for 20 hours, filter with suction, and recrystallize from diethyl ether three times to obtain O-carboxyl ring anhydride. Spectra and carbon spectra are characterized as follows.
[0104] (1) D-ManOCA: 1 H NMR (300MHz, CDCl 3 ):δ7.42-7.53(m,5H),6.02(s,1H). 13 C NMR (300MHz, CDCl 3 ): δ165.46, 148.15, 130.92, 129.67, 129.34, 126.26, 80.55. L-ManOCA: 1 HNMR and 13 C NMR is consistent with D-ManOCA.
[0105] (2)L-LacOCA: 1 H NMR (300MHz, CDCl 3 ):δ5.12(q,1H),1.71(d,3H). 13 C NMR (300MHz, CDCl 3 ): δ167.76, 148.20, 76.29, 16.60.
[0106] (3) L-Ser(Bn)OCA: 1 H NMR (300MHz, CDCl 3 ):δ7.26-7.40(m,5H),5.11(m,1H),4.60(m,2H,),3.91(m,2H,). 13 C NMR (CDCl 3 ,300MHz): δ165.66,148.55, 136.33,128.70,128.32,127.74,79.68,73.75,66.15.
[0107] ...
preparation Embodiment 1
[0119] The preparation of preparation example 1 catalyst 5
[0120]
[0121] Specific steps are as follows:
[0122] (1) Add 5.0g (33.8mmol) of 2,6-dichloropyridine, 643mg (3.38mmol) of copper iodide, and 587mg (6.76mmol) of lithium bromide to the Schlenk bottle, blow nitrogen for 30 minutes, and dissolve the mixture in 20mL at 0°C Anhydrous tetrahydrofuran, keep the temperature, dropwise add a tetrahydrofuran solution of tert-butylmagnesium chloride (50mL, 1M, 50mmol), stir for 12 hours, allow to gradually return to room temperature. After the reaction was completed, post-treatment was performed to obtain 2-tert-butyl-6-chloropyridine with a yield of 70%. With deuterated chloroform (CDCl 3 ) as a reagent with a 500 MHz nuclear magnetic resonance instrument (hydrogen spectrum, 1 H NMR), 125 MHz nuclear magnetic resonance (carbon spectrum, 13 C NMR) and high-resolution mass spectrometry characterized the structure of 2-tert-butyl-6-chloropyridine. 1 H NMR (500MHz, CDCl ...
preparation Embodiment 2
[0127] Preparation of Example 2 Catalyst 7
[0128]
[0129] Specific steps are as follows:
[0130] (1) Add 5.50 g (34 mmol) of 2,3-dichloro-5-methylpyridine, 1.30 g (6.8 mmol) of copper iodide, and 1.20 g (13.4 mmol) of lithium bromide to a Schlenk bottle, and pass nitrogen gas for 30 minutes, and the mixture Dissolve in 25mL of anhydrous tetrahydrofuran at 0°C, keep the temperature, add tert-butylmagnesium chloride solution in tetrahydrofuran (50mL, 1M, 50mmol) dropwise, stir for 12 hours, allow to gradually return to room temperature, and then reflux for 24h. After the reaction was completed, post-treatment was performed to obtain 2-tert-butyl-3-chloro-5-methylpyridine with a yield of 20%. With deuterated chloroform (CDCl 3 ) as a reagent with a 500 MHz NMR instrument (hydrogen spectrum, 1 H NMR) characterized the structure of 2-tert-butyl-3-chloro-5-methylpyridine. 1 H NMR (500MHz, CDCl 3 ):δ8.24(d,1H),7.44(d,1H),2.28(s,3H),1.48(s,9H).
[0131] (2) Add 0.90 g (4....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| tacticity | aaaaa | aaaaa |
| tacticity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com