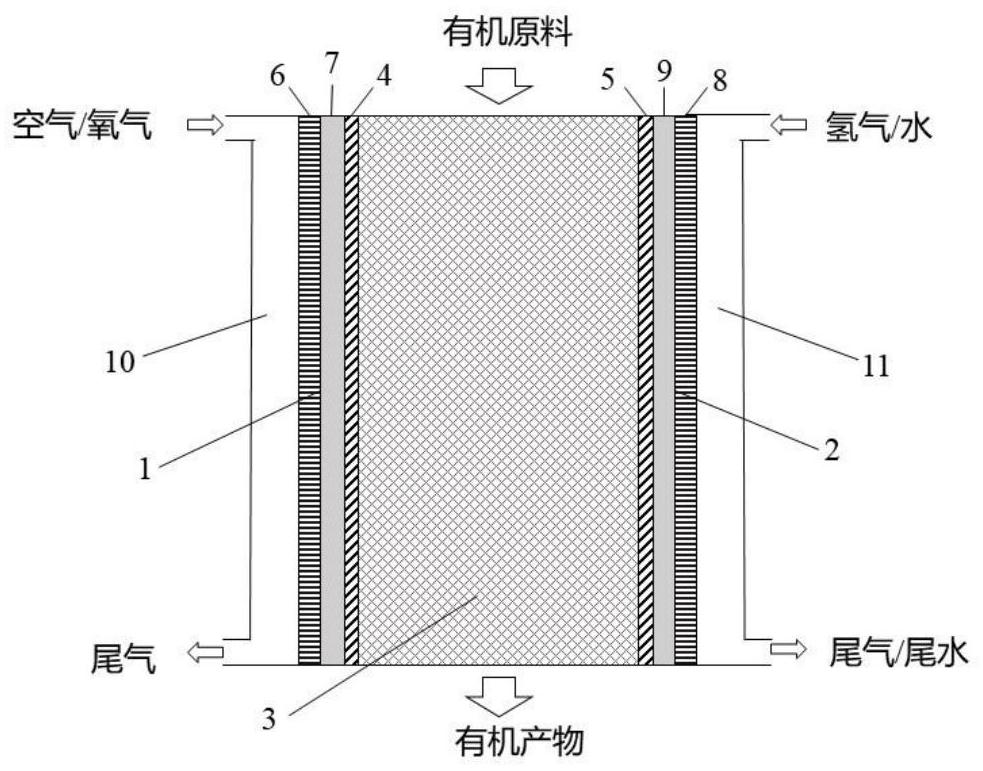
Device and method for coupled electro-catalytic hydrogen peroxide production and selective organic matter oxidation
A technology for organic matter and hydrogen oxidation, which is applied in chemical instruments and methods, oxidation preparation of carbonyl compounds, organic chemistry, etc. It can solve the problems of storage and transportation of hydrogen peroxide, low concentration of hydrogen peroxide, and low reaction current density, etc., so as to reduce equipment investment and Operating costs, broad prospects for industrial applications, and the effect of solving safety problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
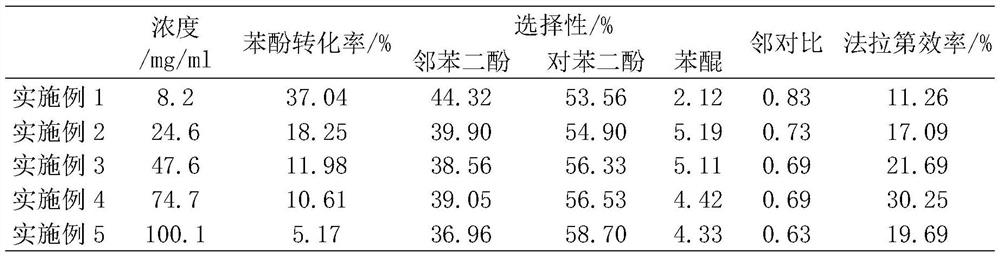
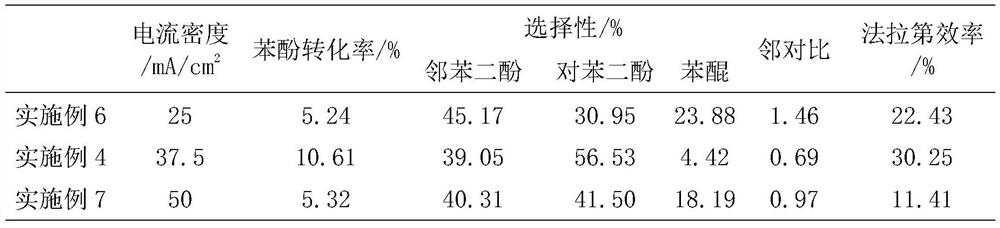
[0029] Using air and hydrogen system, the anode and cathode are 0.5mg / cm 2 Functionalized carbon powder and commercial Pt / C with a catalyst active area of 4 cm 2 , the organic oxidation catalyst is 0.1g TS-1 molecular sieve. 0.1M K 2 SO 4 The aqueous solution is introduced into the organic catalytic chamber through a peristaltic pump for circulation, and the flow rate is maintained at 0.18ml / min, and the power is turned on. At a current density of 37.5mA / cm 2 Under constant current electrolysis, after 30 minutes of activation to stabilize the system, the solution was collected to titrate the concentration of hydrogen peroxide with potassium iodide; organic raw materials (containing 0.085M phenol, 0.1M K2 SO 4 ) is introduced into the organic catalytic chamber through a peristaltic pump, the total volume of the solution is 20ml, and the flow rate is 0.18ml / min; when the current density is 37.5mA / cm 2 During constant current electrolysis at room temperature, the cell volta...
Embodiment 2
[0031] This example is basically the same as Example 1, except that the concentration of the raw material phenol is enlarged to 0.262M. After the activation is stable, 20ml containing 0.262M phenol, 0.1M K 2 SO 4 The aqueous solution is introduced into the organic catalytic chamber through a peristaltic pump, and at a current density of 37.5mA / cm 2 Under the constant current solution cycle electrolysis for 2h, the cell voltage is about 0.85V, and then power off cycle for 8h. The conversion rate of phenol is about 18.25%, the selectivity of hydroquinone is 94.81%, the ratio of catechol to hydroquinone is 0.73, and the current efficiency is 17.09%.
Embodiment 3
[0033] This example is basically the same as Example 1, except that the concentration of the raw material phenol is enlarged to 0.506M. After the activation is stable, 20ml containing 0.506M phenol, 0.1M K 2 SO 4 The aqueous solution is introduced into the organic catalytic chamber through a peristaltic pump, and at a current density of 37.5mA / cm 2 Under the constant current solution cycle electrolysis for 2h, the cell voltage is about 0.9V, and then cut off the power cycle for 8h. The conversion rate of phenol is about 11.98%, the selectivity of hydroquinone is 94.89%, the ratio of catechol to hydroquinone is 0.69, and the current efficiency is 21.69%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com