Modified diaphragm for lithium-sulfur battery and preparation process of modified diaphragm
A technology of lithium-sulfur battery and preparation process, which is applied in the direction of lithium storage battery, battery pack parts, non-aqueous electrolyte storage battery, etc. It can solve the problems of active material loss, discharge capacity attenuation, failure to meet high specific capacity and high energy density, etc. Achieve the effects of improving Coulombic efficiency and cycle life, improving mechanical stability, and inhibiting the shuttle effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
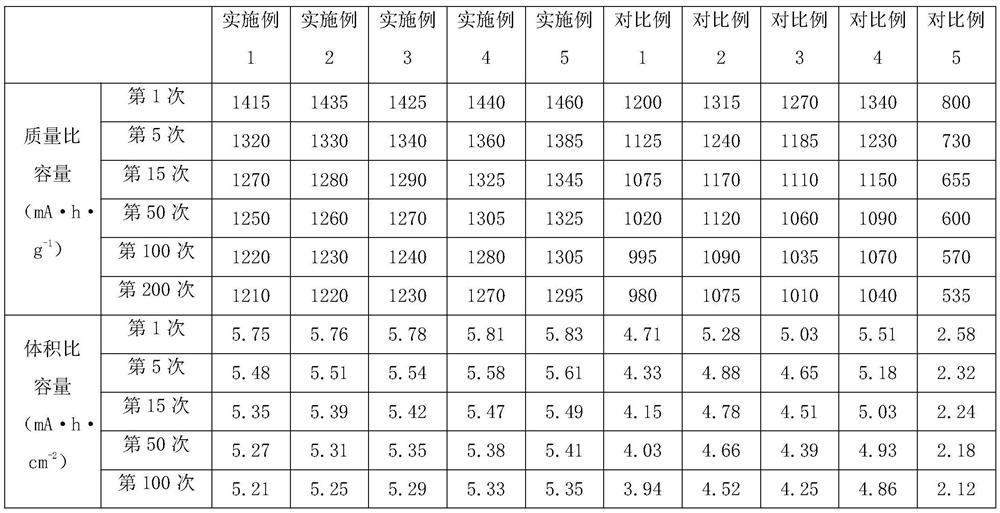
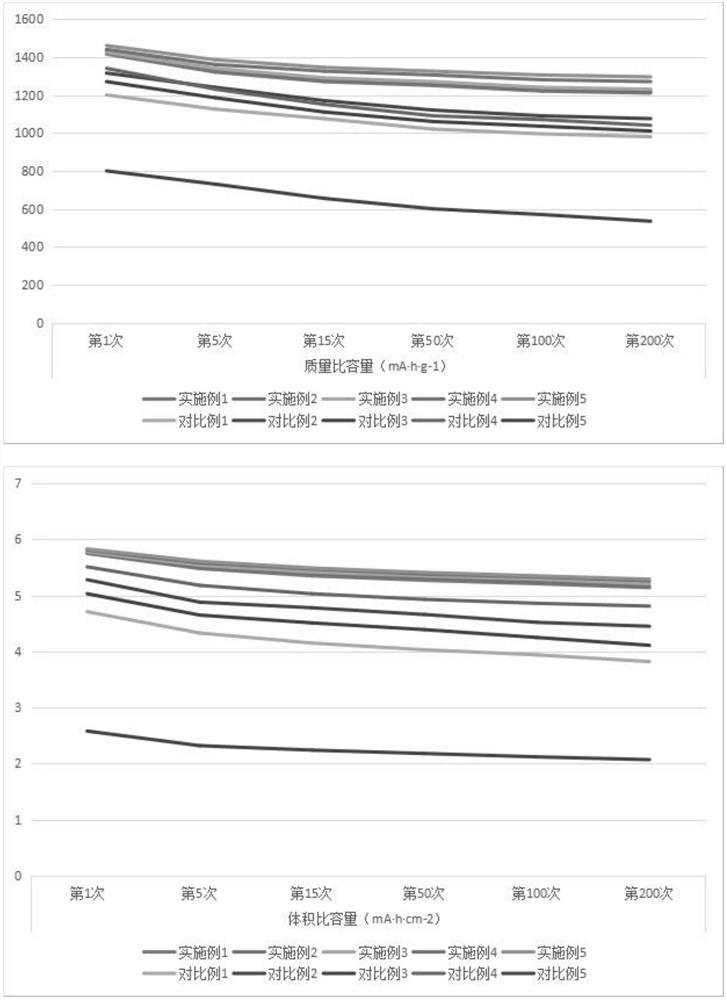
Embodiment 1
[0030] (1) Dissolve cobalt nitrate and dimethylimidazole in deionized water, the molar ratio of cobalt nitrate and dimethylimidazole is 1:1, place it at a temperature of 30°C for reaction, and the reaction time is 5h. After the reaction is completed, Form a precursor, add a binder, the binder is styrene-butadiene rubber, and prepare a precursor slurry;
[0031] (2) Take the precursor slurry and apply it on the surface of the base film, and place it at 60°C for drying for 0.5 hours to form a precursor coating and obtain a separator A;
[0032] (3) Take diaphragm A, place it in an atomic deposition instrument, and use 0.01mol / L aluminum chloride and deionized water as reaction sources to deposit on the surface of the precursor coating to form a nano-metal oxide layer, in which the nano-metal Oxide is Al 2 o 3 , deposition process parameters: the reaction temperature is 40°C, the atomic layer deposition cycle is 20 weeks, and the membrane B is obtained;
[0033] (4) Take the d...
Embodiment 2
[0035] (1) Dissolve cobalt nitrate and dimethylimidazole in deionized water, the molar ratio of cobalt nitrate and dimethylimidazole is 1:1, and react at a temperature of 55°C. The reaction time is 8.5h, and the reaction is completed , form a precursor, add a binder, the binder is polyacrylic acid, and prepare a precursor slurry;
[0036] (2) Take the precursor slurry and apply it on the surface of the base film, and dry it at 70°C for 1 hour to form a precursor coating and obtain a separator A;
[0037] (3) Take diaphragm A, place it in an atomic deposition instrument, and use 0.01mol / L ruthenium chloride and deionized water as reaction sources to deposit on the surface of the precursor coating to form a nano-metal oxide layer, wherein the nano-metal Oxide is RuO 2 , deposition process parameters: the reaction temperature is 60°C, the atomic layer deposition cycle is 35 weeks, and the diaphragm B is obtained;
[0038] (4) Take the diaphragm B, place it in a carbon disulfide...
Embodiment 3
[0040] (1) Dissolve cobalt nitrate and dimethylimidazole in deionized water, the molar ratio of cobalt nitrate and dimethylimidazole is 1:1, place it at a temperature of 80°C for reaction, and the reaction time is 12h. After the reaction is completed, Form a precursor, add a binder, the binder is polyvinylidene fluoride, and prepare a precursor slurry;
[0041] (2) Take the precursor slurry and apply it on the surface of the base film, and place it at 80°C for drying for 1 hour to form a precursor coating and obtain a separator A;
[0042](3) Take diaphragm A, place it in an atomic deposition instrument, and use 0.01mol / L manganese chloride and deionized water as reaction sources to deposit on the surface of the precursor coating to form a nano-metal oxide layer, wherein the nano-metal The oxide is MnO, the deposition process parameters: the reaction temperature is 80°C, the atomic layer deposition cycle is 50 weeks, and the diaphragm B is obtained;
[0043] (4) Take the diap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com